Basic residues within the ebolavirus VP35 protein are required for its viral polymerase cofactor function
- PMID: 20686031
- PMCID: PMC2950600
- DOI: 10.1128/JVI.00925-10
Basic residues within the ebolavirus VP35 protein are required for its viral polymerase cofactor function
Abstract
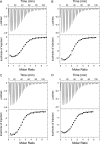
The ebolavirus (EBOV) VP35 protein binds to double-stranded RNA (dsRNA), inhibits host alpha/beta interferon (IFN-α/β) production, and is an essential component of the viral polymerase complex. Structural studies of the VP35 C-terminal IFN inhibitory domain (IID) identified specific structural features, including a central basic patch and a hydrophobic pocket, that are important for dsRNA binding and IFN inhibition. Several other conserved basic residues bordering the central basic patch and a separate cluster of basic residues, called the first basic patch, were also identified. Functional analysis of alanine substitution mutants indicates that basic residues outside the central basic patch are not required for dsRNA binding or for IFN inhibition. However, minigenome assays, which assess viral RNA polymerase complex function, identified these other basic residues to be critical for viral RNA synthesis. Of these, a subset located within the first basic patch is important for VP35-nucleoprotein (NP) interaction, as evidenced by the inability of alanine substitution mutants to coimmunoprecipitate with NP. Therefore, first basic patch residues are likely critical for replication complex formation through interactions with NP. Coimmunoprecipitation studies further demonstrate that the VP35 IID is sufficient to interact with NP and that dsRNA can modulate VP35 IID interactions with NP. Other basic residue mutations that disrupt the VP35 polymerase cofactor function do not affect interaction with NP or with the amino terminus of the viral polymerase. Collectively, these results highlight the importance of conserved basic residues from the EBOV VP35 C-terminal IID and validate the VP35 IID as a potential therapeutic target.
Figures





References
-
- Becker, S., C. Rinne, U. Hofsass, H. D. Klenk, and E. Muhlberger. 1998. Interactions of Marburg virus nucleocapsid proteins. Virology 249:406-417. - PubMed
-
- Boehmann, Y., S. Enterlein, A. Randolf, and E. Muhlberger. 2005. A reconstituted replication and transcription system for Ebola virus Reston and comparison with Ebola virus Zaire. Virology 332:406-417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI081914/AI/NIAID NIH HHS/United States
- 1F32AI084453/AI/NIAID NIH HHS/United States
- U54 AI057160/AI/NIAID NIH HHS/United States
- F32 AI084453/AI/NIAID NIH HHS/United States
- R56 AI089547/AI/NIAID NIH HHS/United States
- F32 AI084324/AI/NIAID NIH HHS/United States
- R01AI081914/AI/NIAID NIH HHS/United States
- 1R56AI089547/AI/NIAID NIH HHS/United States
- R01AI059536/AI/NIAID NIH HHS/United States
- U54AI057160/AI/NIAID NIH HHS/United States
- R01 AI059536/AI/NIAID NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
- AI057158/AI/NIAID NIH HHS/United States
- 1F32AI084324/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

