Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture
- PMID: 20686033
- PMCID: PMC2953183
- DOI: 10.1128/JVI.00526-10
Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture
Abstract
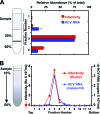
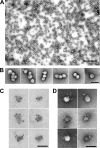
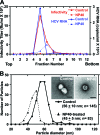
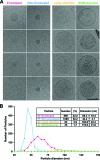
We analyzed the biochemical and ultrastructural properties of hepatitis C virus (HCV) particles produced in cell culture. Negative-stain electron microscopy revealed that the particles were spherical (∼40- to 75-nm diameter) and pleomorphic and that some of them contain HCV E2 protein and apolipoprotein E on their surfaces. Electron cryomicroscopy revealed two major particle populations of ∼60 and ∼45 nm in diameter. The ∼60-nm particles were characterized by a membrane bilayer (presumably an envelope) that is spatially separated from an internal structure (presumably a capsid), and they were enriched in fractions that displayed a high infectivity-to-HCV RNA ratio. The ∼45-nm particles lacked a membrane bilayer and displayed a higher buoyant density and a lower infectivity-to-HCV RNA ratio. We also observed a minor population of very-low-density, >100-nm-diameter vesicular particles that resemble exosomes. This study provides low-resolution ultrastructural information of particle populations displaying differential biophysical properties and specific infectivity. Correlative analysis of the abundance of the different particle populations with infectivity, HCV RNA, and viral antigens suggests that infectious particles are likely to be present in the large ∼60-nm HCV particle populations displaying a visible bilayer. Our study constitutes an initial approach toward understanding the structural characteristics of infectious HCV particles.
Figures


References
-
- Atshaves, B. P., A. L. McIntosh, H. R. Payne, A. M. Gallegos, K. Landrock, N. Maeda, A. B. Kier, and F. Schroeder. 2007. SCP-2/SCP-x gene ablation alters lipid raft domains in primary cultured mouse hepatocytes. J. Lipid Res. 48:2193-2211. - PubMed
-
- Baumert, T. F., J. Vergalla, J. Satoi, M. Thomson, M. Lechmann, D. Herion, H. B. Greenberg, S. Ito, and T. J. Liang. 1999. Hepatitis C virus-like particles synthesized in insect cells as a potential vaccine candidate. Gastroenterology 117:1397-1407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

