Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae
- PMID: 20686035
- PMCID: PMC2950567
- DOI: 10.1128/JVI.00450-10
Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae
Abstract
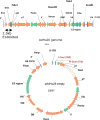
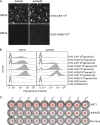
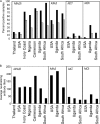
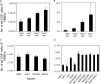
In order to better understand the broad applicability of adenovirus (Ad) as a vector for human vaccine studies, we compared four adenovirus (Ad) vectors from families C (Ad human serotype 5 [HAdV-5; here referred to as AdHu5]), D (HAdV-26; here referred to as AdHu26), and E (simian serotypes SAdV-23 and SAdV-24; here referred to as chimpanzee serotypes 6 and 7 [AdC6 and AdC7, respectively]) of the Adenoviridae. Seroprevalence rates and titers of neutralizing antibodies to the two human-origin Ads were found to be higher than those reported previously, especially in countries of sub-Saharan Africa. Conversely, prevalence rates and titers to AdC6 and AdC7 were markedly lower. Healthy human adults from the United States had readily detectable circulating T cells recognizing Ad viruses, the levels of which in some individuals were unexpectedly high in response to AdHu26. The magnitude of T-cell responses to AdHu5 correlated with those to AdHu26, suggesting T-cell recognition of conserved epitopes. In mice, all of the different Ad vectors induced CD8(+) T-cell responses that were comparable in their magnitudes and cytokine production profiles. Prime-boost regimens comparing different combinations of Ad vectors failed to indicate that the sequential use of Ad vectors from distinct families resulted in higher immune responses than the use of serologically distinct Ad vectors from the same family. Moreover, the transgene product-specific antibody responses induced by the AdHu26 and AdC vectors were markedly lower than those induced by the AdHu5 vector. AdHu26 vectors and, to a lesser extent, AdC vectors induced more potent Ad-neutralizing antibody responses. These results suggest that the potential of AdHu26 as a vaccine vector may suffer from limitations similar to those found for vectors based on other prevalent human Ads.
Figures


Similar articles
-
Neutralizing antibodies to human and simian adenoviruses in humans and New-World monkeys.Virology. 2010 Nov 10;407(1):1-6. doi: 10.1016/j.virol.2010.07.043. Epub 2010 Aug 24. Virology. 2010. PMID: 20797754
-
Seroprevalence of neutralizing antibodies to human adenoviruses type-5 and type-26 and chimpanzee adenovirus type-68 in healthy Chinese adults.J Med Virol. 2013 Jun;85(6):1077-84. doi: 10.1002/jmv.23546. J Med Virol. 2013. PMID: 23588735
-
Generation of an adenoviral vaccine vector based on simian adenovirus 21.J Gen Virol. 2006 Sep;87(Pt 9):2477-2485. doi: 10.1099/vir.0.81989-0. J Gen Virol. 2006. PMID: 16894185
-
Adenovirus vectors composed of subgroup B adenoviruses.Curr Gene Ther. 2007 Aug;7(4):229-38. doi: 10.2174/156652307781369137. Curr Gene Ther. 2007. PMID: 17969556 Review.
-
The seroprevalence of adenoviruses since 20001.Emerg Microbes Infect. 2025 Dec;14(1):2475831. doi: 10.1080/22221751.2025.2475831. Epub 2025 Mar 17. Emerg Microbes Infect. 2025. PMID: 40035700 Free PMC article. Review.
Cited by
-
Analysis of the Prevalence of Binding and Neutralizing Antibodies against 39 Human Adenovirus Types in Student Cohorts Reveals Low-Prevalence Types and a Decline in Binding Antibody Levels during the SARS-CoV-2 Pandemic.J Virol. 2022 Nov 23;96(22):e0113322. doi: 10.1128/jvi.01133-22. Epub 2022 Nov 7. J Virol. 2022. PMID: 36342295 Free PMC article.
-
Human Adenovirus Type 26 Infection Mediated by αvβ3 Integrin Is Caveolin-1-Dependent.Microbiol Spectr. 2022 Aug 31;10(4):e0109722. doi: 10.1128/spectrum.01097-22. Epub 2022 Aug 4. Microbiol Spectr. 2022. PMID: 35924932 Free PMC article.
-
A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity.PLoS One. 2012;7(7):e40385. doi: 10.1371/journal.pone.0040385. Epub 2012 Jul 13. PLoS One. 2012. PMID: 22808149 Free PMC article.
-
Controlling the HIV/AIDS epidemic: current status and global challenges.Front Immunol. 2012 Aug 14;3:250. doi: 10.3389/fimmu.2012.00250. eCollection 2012. Front Immunol. 2012. PMID: 22912636 Free PMC article.
-
Immunological and virological analyses of rhesus macaques immunized with chimpanzee adenoviruses expressing the simian immunodeficiency virus Gag/Tat fusion protein and challenged intrarectally with repeated low doses of SIVmac.J Virol. 2013 Sep;87(17):9420-30. doi: 10.1128/JVI.01456-13. Epub 2013 Jun 26. J Virol. 2013. PMID: 23804645 Free PMC article.
References
-
- Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. - PMC - PubMed
-
- Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. - PMC - PubMed
-
- Bansal, G. P., A. Malaspina, and J. Flores. Future paths for HIV vaccine research: exploiting results from recent clinical trials and current scientific advances. Curr. Opin. Mol. Ther. 12:39-46. - PubMed
-
- Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. - PMC - PubMed
-
- Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416-1422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

