Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate
- PMID: 20686041
- PMCID: PMC2950575
- DOI: 10.1128/JVI.00614-10
Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate
Abstract
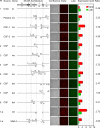
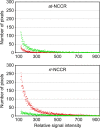
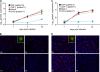
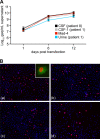
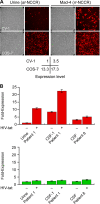
Polyomavirus JC (JCV) infects ∼ 60% of the general population, followed by asymptomatic urinary shedding in ∼ 20%. In patients with pronounced immunodeficiency, including HIV/AIDS, JCV can cause progressive multifocal leukoencephalopathy (PML), a devastating brain disease of high mortality. While JCV in the urine of healthy people has a linear noncoding control region called the archetype NCCR (at-NCCR), JCV in brain and cerebrospinal fluid (CSF) of PML patients bear rearranged NCCRs (rr-NCCRs). Although JCV NCCR rearrangements are deemed pathognomonic for PML, their role as a viral determinant is unclear. We sequenced JCV NCCRs found in CSF of eight HIV/AIDS patients newly diagnosed with PML and analyzed their effect on early and late gene expression using a bidirectional reporter vector recapitulating the circular polyomavirus early and late gene organization. The rr-NCCR sequences were highly diverse, but all increased viral early reporter gene expression in progenitor-derived astrocytes, glia-derived cells, and human kidney compared to the expression levels with the at-NCCR. The expression of simian virus 40 (SV40) large T antigen or HIV Tat expression in trans was associated with a strong increase of at-NCCR-controlled early gene expression, while rr-NCCRs were less responsive. The insertion of rr-NCCRs into the JCV genome backbone revealed higher viral replication rates for rr-NCCR compared to those of the at-NCCR JCV in human progenitor-derived astrocytes or glia cells, which was abrogated in SV40 large T-expressing COS-7 cells. We conclude that naturally occurring JCV rr-NCCR variants from PML patients confer increased early gene expression and higher replication rates compared to those of at-NCCR JCV and thereby increase cytopathology.
Figures
Similar articles
-
High-resolution melting analysis for mutation scanning in the non-coding control region of JC polyomavirus from patients with progressive multifocal leukoencephalopathy.Arch Virol. 2014 Jul;159(7):1687-96. doi: 10.1007/s00705-014-1988-4. Epub 2014 Jan 25. Arch Virol. 2014. PMID: 24463953
-
Identical rearranged forms of JC polyomavirus transcriptional control region in plasma and cerebrospinal fluid of acquired immunodeficiency syndrome patients with progressive multifocal leukoencephalopathy.J Neurovirol. 2003 Oct;9(5):551-8. doi: 10.1080/13550280390241188. J Neurovirol. 2003. PMID: 13129769
-
Sequential changes in the non-coding control region sequences of JC polyomaviruses from the cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy.Arch Virol. 2013 Mar;158(3):639-50. doi: 10.1007/s00705-012-1532-3. Epub 2012 Nov 9. Arch Virol. 2013. PMID: 23138154
-
Polyomavirus JC in the context of immunosuppression: a series of adaptive, DNA replication-driven recombination events in the development of progressive multifocal leukoencephalopathy.Clin Dev Immunol. 2013;2013:197807. doi: 10.1155/2013/197807. Epub 2013 Apr 15. Clin Dev Immunol. 2013. PMID: 23690820 Free PMC article. Review.
-
[New approach for JC virus detection and its application for PML diagnosis].Rinsho Shinkeigaku. 2014;54(12):1028-30. doi: 10.5692/clinicalneurol.54.1028. Rinsho Shinkeigaku. 2014. PMID: 25672699 Review. Japanese.
Cited by
-
A Difficult Decision: Atypical JC Polyomavirus Encephalopathy in a Kidney Transplant Recipient.Transplantation. 2017 Jun;101(6):1461-1467. doi: 10.1097/TP.0000000000001275. Transplantation. 2017. PMID: 27367472 Free PMC article.
-
Transcription factor Spi-B binds unique sequences present in the tandem repeat promoter/enhancer of JC virus and supports viral activity.J Gen Virol. 2010 Dec;91(Pt 12):3042-52. doi: 10.1099/vir.0.023184-0. Epub 2010 Sep 8. J Gen Virol. 2010. PMID: 20826618 Free PMC article.
-
Structural Analysis of Merkel Cell Polyomavirus (MCPyV) Viral Capsid Protein 1 (VP1) in HIV-1 Infected Individuals.Int J Mol Sci. 2020 Oct 27;21(21):7998. doi: 10.3390/ijms21217998. Int J Mol Sci. 2020. PMID: 33121182 Free PMC article.
-
Progressive Multifocal Leukoencephalopathy during Tocilizumab Treatment for Rheumatoid Arthritis.Intern Med. 2020 Aug 15;59(16):2053-2059. doi: 10.2169/internalmedicine.4431-20. Epub 2020 May 23. Intern Med. 2020. PMID: 32448834 Free PMC article.
-
The raccoon polyomavirus genome and tumor antigen transcription are stable and abundant in neuroglial tumors.J Virol. 2014 Nov;88(21):12816-24. doi: 10.1128/JVI.01912-14. Epub 2014 Aug 27. J Virol. 2014. PMID: 25165109 Free PMC article.
References
-
- Agostini, H. T., C. F. Ryschkewitsch, E. J. Singer, and G. L. Stoner. 1997. JC virus regulatory region rearrangements and genotypes in progressive multifocal leukoencephalopathy: two independent aspects of virus variation. J. Gen. Virol. 78:659-664. - PubMed
-
- Albrecht, H., C. Hoffmann, O. Degen, A. Stoehr, A. Plettenberg, T. Mertenskotter, C. Eggers, and H. J. Stellbrink. 1998. Highly active antiretroviral therapy significantly improves the prognosis of patients with HIV-associated progressive multifocal leukoencephalopathy. AIDS 12:1149-1154. - PubMed
-
- Astrom, K. E., E. L. Mancall, and E. P. Richardson, Jr. 1958. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain 81:93-111. - PubMed
-
- Ault, G. S., and G. L. Stoner. 1993. Human polyomavirus JC promoter/enhancer rearrangement patterns from progressive multifocal leukoencephalopathy brain are unique derivatives of a single archetypal structure. J. Gen. Virol. 74:1499-1507. - PubMed
-
- Berger, J. R., B. Kaszovitz, M. J. Post, and G. Dickinson. 1987. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann. Intern. Med. 107:78-87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

