Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate
- PMID: 20686041
- PMCID: PMC2950575
- DOI: 10.1128/JVI.00614-10
Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate
Abstract
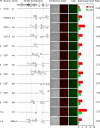
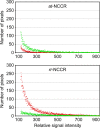
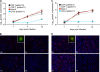
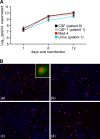
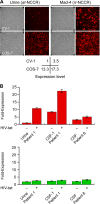
Polyomavirus JC (JCV) infects ∼ 60% of the general population, followed by asymptomatic urinary shedding in ∼ 20%. In patients with pronounced immunodeficiency, including HIV/AIDS, JCV can cause progressive multifocal leukoencephalopathy (PML), a devastating brain disease of high mortality. While JCV in the urine of healthy people has a linear noncoding control region called the archetype NCCR (at-NCCR), JCV in brain and cerebrospinal fluid (CSF) of PML patients bear rearranged NCCRs (rr-NCCRs). Although JCV NCCR rearrangements are deemed pathognomonic for PML, their role as a viral determinant is unclear. We sequenced JCV NCCRs found in CSF of eight HIV/AIDS patients newly diagnosed with PML and analyzed their effect on early and late gene expression using a bidirectional reporter vector recapitulating the circular polyomavirus early and late gene organization. The rr-NCCR sequences were highly diverse, but all increased viral early reporter gene expression in progenitor-derived astrocytes, glia-derived cells, and human kidney compared to the expression levels with the at-NCCR. The expression of simian virus 40 (SV40) large T antigen or HIV Tat expression in trans was associated with a strong increase of at-NCCR-controlled early gene expression, while rr-NCCRs were less responsive. The insertion of rr-NCCRs into the JCV genome backbone revealed higher viral replication rates for rr-NCCR compared to those of the at-NCCR JCV in human progenitor-derived astrocytes or glia cells, which was abrogated in SV40 large T-expressing COS-7 cells. We conclude that naturally occurring JCV rr-NCCR variants from PML patients confer increased early gene expression and higher replication rates compared to those of at-NCCR JCV and thereby increase cytopathology.
Figures
References
-
- Agostini, H. T., C. F. Ryschkewitsch, E. J. Singer, and G. L. Stoner. 1997. JC virus regulatory region rearrangements and genotypes in progressive multifocal leukoencephalopathy: two independent aspects of virus variation. J. Gen. Virol. 78:659-664. - PubMed
-
- Albrecht, H., C. Hoffmann, O. Degen, A. Stoehr, A. Plettenberg, T. Mertenskotter, C. Eggers, and H. J. Stellbrink. 1998. Highly active antiretroviral therapy significantly improves the prognosis of patients with HIV-associated progressive multifocal leukoencephalopathy. AIDS 12:1149-1154. - PubMed
-
- Astrom, K. E., E. L. Mancall, and E. P. Richardson, Jr. 1958. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain 81:93-111. - PubMed
-
- Ault, G. S., and G. L. Stoner. 1993. Human polyomavirus JC promoter/enhancer rearrangement patterns from progressive multifocal leukoencephalopathy brain are unique derivatives of a single archetypal structure. J. Gen. Virol. 74:1499-1507. - PubMed
-
- Berger, J. R., B. Kaszovitz, M. J. Post, and G. Dickinson. 1987. Progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. A review of the literature with a report of sixteen cases. Ann. Intern. Med. 107:78-87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

