Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin
- PMID: 20686043
- PMCID: PMC2950602
- DOI: 10.1128/JVI.00103-10
Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin
Abstract
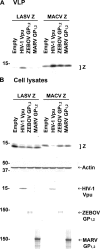
Bone marrow stromal antigen 2 (BST-2/tetherin) is a cellular membrane protein that inhibits the release of HIV-1. We show for the first time, using infectious viruses, that BST-2 also inhibits egress of arenaviruses but has no effect on filovirus replication and spread. Specifically, infectious Lassa virus (LASV) release significantly decreased or increased in human cells in which BST-2 was either stably expressed or knocked down, respectively. In contrast, replication and spread of infectious Zaire ebolavirus (ZEBOV) and Lake Victoria marburgvirus (MARV) were not affected by these conditions. Replication of infectious Rift Valley fever virus (RVFV) and cowpox virus (CPXV) was also not affected by BST-2 expression. Elevated cellular levels of human or murine BST-2 inhibited the release of virus-like particles (VLPs) consisting of the matrix proteins of multiple highly virulent NIAID Priority Pathogens, including arenaviruses (LASV and Machupo virus [MACV]), filoviruses (ZEBOV and MARV), and paramyxoviruses (Nipah virus). Although the glycoproteins of filoviruses counteracted the antiviral activity of BST-2 in the context of VLPs, they could not rescue arenaviral (LASV and MACV) VLP release upon BST-2 overexpression. Furthermore, we did not observe colocalization of filoviral glycoproteins with BST-2 during infection with authentic viruses. None of the arenavirus-encoded proteins rescued budding of VLPs in the presence of BST-2. Our results demonstrate that BST-2 might be a broad antiviral factor with the ability to restrict release of a wide variety of human pathogens. However, at least filoviruses, RVFV, and CPXV are immune to its inhibitory effect.
Figures




Similar articles
-
Analysis of determinants in filovirus glycoproteins required for tetherin antagonism.Viruses. 2014 Apr 9;6(4):1654-71. doi: 10.3390/v6041654. Viruses. 2014. PMID: 24721789 Free PMC article.
-
Human BST-2/tetherin inhibits Junin virus release from host cells and its inhibition is partially counteracted by viral nucleoprotein.J Gen Virol. 2020 Jun;101(6):573-586. doi: 10.1099/jgv.0.001414. Epub 2020 Apr 24. J Gen Virol. 2020. PMID: 32375950
-
Hemorrhagic Fever Virus Budding Studies.Methods Mol Biol. 2018;1604:209-215. doi: 10.1007/978-1-4939-6981-4_15. Methods Mol Biol. 2018. PMID: 28986836
-
Molecular virulence determinants of human-pathogenic filoviruses.Adv Virus Res. 2025;121:1-29. doi: 10.1016/bs.aivir.2025.03.003. Epub 2025 Apr 2. Adv Virus Res. 2025. PMID: 40379380 Review.
-
Limiting Respiratory Viral Infection by Targeting Antiviral and Immunological Functions of BST-2/Tetherin: Knowledge and Gaps.Bioessays. 2018 Oct;40(10):e1800086. doi: 10.1002/bies.201800086. Epub 2018 Aug 16. Bioessays. 2018. PMID: 30113067 Free PMC article. Review.
Cited by
-
Crosstalk Between SUMO and Ubiquitin-Like Proteins: Implication for Antiviral Defense.Front Cell Dev Biol. 2021 Apr 21;9:671067. doi: 10.3389/fcell.2021.671067. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33968942 Free PMC article. Review.
-
Analysis of determinants in filovirus glycoproteins required for tetherin antagonism.Viruses. 2014 Apr 9;6(4):1654-71. doi: 10.3390/v6041654. Viruses. 2014. PMID: 24721789 Free PMC article.
-
A host-oriented inhibitor of Junin Argentine hemorrhagic fever virus egress.J Virol. 2014 May;88(9):4736-43. doi: 10.1128/JVI.03757-13. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522922 Free PMC article.
-
DDX3 suppresses type I interferons and favors viral replication during Arenavirus infection.PLoS Pathog. 2018 Jul 12;14(7):e1007125. doi: 10.1371/journal.ppat.1007125. eCollection 2018 Jul. PLoS Pathog. 2018. PMID: 30001425 Free PMC article.
-
Three-Dimensional Structural Characterization of HIV-1 Tethered to Human Cells.J Virol. 2015 Nov 18;90(3):1507-21. doi: 10.1128/JVI.01880-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26582000 Free PMC article.
References
-
- Baize, S., J. Kaplon, C. Faure, D. Pannetier, M. C. Georges-Courbot, and V. Deubel. 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 172:2861-2869. - PubMed
-
- Baize, S., D. Pannetier, C. Faure, P. Marianneau, I. Marendat, M. C. Georges-Courbot, and V. Deubel. 2006. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect. 8:1194-1202. - PubMed
-
- Battles, J. K., and J. M. Dalrymple. 1988. Genetic variation among geographic isolates of Rift Valley fever virus. Am. J. Trop. Med. Hyg. 39:617-631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

