Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16
- PMID: 20686044
- PMCID: PMC2950566
- DOI: 10.1128/JVI.00552-10
Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16
Abstract
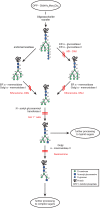
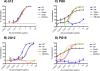
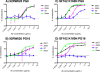
The HIV-1-specific antibodies PG9 and PG16 show marked cross-isolate neutralization breadth and potency. Antibody neutralization has been shown to be dependent on the presence of N-linked glycosylation at position 160 in gp120. We show here that (i) the loss of several key glycosylation sites in the V1, V2, and V3 loops; (ii) the generation of pseudoviruses in the presence of various glycosidase inhibitors; and (iii) the growth of pseudoviruses in a mutant cell line (GnT1(-/-)) that alters envelope glycosylation patterns all have significant effects on the sensitivity of virus to neutralization by PG9 and PG16. However, the interaction of antibody is not inhibited by sugar monosaccharides corresponding to those found in glycans on the HIV surface. We show that some of the glycosylation effects described are isolate dependent and others are universal and can be used as diagnostic for the presence of PG9 and PG16-like antibodies in the sera of HIV-1-infected patients. The results suggest that PG9 and PG16 recognize a conformational epitope that is dependent on glycosylation at specific variable loop N-linked sites. This information may be valuable for the design of immunogens to elicit PG9 and PG16-like antibodies, as well as constructs for cocrystallization studies.
Figures



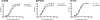

References
-
- Ben-Dor, S., N. Esterman, E. Rubin, and N. Sharon. 2004. Biases and complex patterns in the residues flanking protein N glycosylation sites. Glycobiology 14:95-101. - PubMed
-
- Burton, D. R. 2002. Antibodies, viruses, and vaccines. Nat. Rev. Immunol. 2:706-713. - PubMed
-
- Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. - PubMed
-
- Calarese, D. A., C. N. Scanlan, M. B. Zwick, S. Deechongkit, Y. Mimura, R. Kunert, P. Zhu, M. R. Wormald, R. L. Stanfield, K. H. Roux, J. W. Kelly, P. M. Rudd, R. A. Dwek, H. Katinger, D. R. Burton, and I. A. Wilson. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065-2071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

