Epitope-specific regulatory CD4 T cells reduce virus-induced illness while preserving CD8 T-cell effector function at the site of infection
- PMID: 20686045
- PMCID: PMC2950556
- DOI: 10.1128/JVI.00963-10
Epitope-specific regulatory CD4 T cells reduce virus-induced illness while preserving CD8 T-cell effector function at the site of infection
Abstract
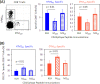
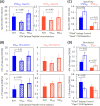
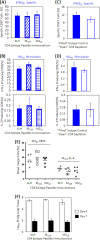
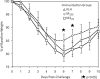
The role of epitope-specific regulatory CD4 T cells in modulating CD8 T-cell-mediated immunopathology during acute viral infection has not been well defined. In the murine model of respiratory syncytial virus (RSV) infection, CD8 T cells play an important role in both viral clearance and immunopathology. We have previously characterized two RSV epitope-specific CD4 T-cell responses with distinct phenotypic properties. One of them, the IA(b)M(209)-specific subset, constitutively expresses FoxP3 and modulates CD8 T-cell function in vitro. We show here that the IA(b)M(209)-specific CD4 T-cell response regulates CD8 T-cell function in vivo and is associated with diminished RSV-induced illness without affecting viral clearance at the site of infection. Achieving the optimal balance of regulatory and effector T-cell function is an important consideration for designing future vaccines.
Figures

Similar articles
-
Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection.J Immunol. 2010 Aug 15;185(4):2382-92. doi: 10.4049/jimmunol.1000423. Epub 2010 Jul 16. J Immunol. 2010. PMID: 20639494 Free PMC article.
-
Determining the breadth of the respiratory syncytial virus-specific T cell response.J Virol. 2014 Mar;88(6):3135-43. doi: 10.1128/JVI.02139-13. Epub 2013 Dec 26. J Virol. 2014. PMID: 24371055 Free PMC article.
-
Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection.J Exp Med. 1997 Aug 4;186(3):421-32. doi: 10.1084/jem.186.3.421. J Exp Med. 1997. PMID: 9236194 Free PMC article.
-
The CD4 T cell response to respiratory syncytial virus infection.Immunol Res. 2014 Aug;59(1-3):109-17. doi: 10.1007/s12026-014-8540-1. Immunol Res. 2014. PMID: 24838148 Review.
-
Cytokines and CD8 T cell immunity during respiratory syncytial virus infection.Cytokine. 2020 Sep;133:154481. doi: 10.1016/j.cyto.2018.07.012. Epub 2018 Jul 18. Cytokine. 2020. PMID: 30031680 Free PMC article. Review.
Cited by
-
A Numerically Subdominant CD8 T Cell Response to Matrix Protein of Respiratory Syncytial Virus Controls Infection with Limited Immunopathology.PLoS Pathog. 2016 Mar 4;12(3):e1005486. doi: 10.1371/journal.ppat.1005486. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 26943673 Free PMC article.
-
Effects of Probiotic Supplementation on Exercise with Predominance of Aerobic Metabolism in Trained Population: A Systematic Review, Meta-Analysis and Meta-Regression.Nutrients. 2022 Jan 30;14(3):622. doi: 10.3390/nu14030622. Nutrients. 2022. PMID: 35276980 Free PMC article.
-
The CD8 T Cell Response to Respiratory Virus Infections.Front Immunol. 2018 Apr 9;9:678. doi: 10.3389/fimmu.2018.00678. eCollection 2018. Front Immunol. 2018. PMID: 29686673 Free PMC article. Review.
-
Legend of the Sentinels: Development of Lung Resident Memory T Cells and Their Roles in Diseases.Front Immunol. 2021 Feb 2;11:624411. doi: 10.3389/fimmu.2020.624411. eCollection 2020. Front Immunol. 2021. PMID: 33603755 Free PMC article. Review.
-
Differential B7-CD28 costimulatory requirements for stable and inflationary mouse cytomegalovirus-specific memory CD8 T cell populations.J Immunol. 2011 Apr 1;186(7):3874-81. doi: 10.4049/jimmunol.1003231. Epub 2011 Feb 28. J Immunol. 2011. PMID: 21357256 Free PMC article.
References
-
- Anz, D., V. H. Koelzer, S. Moder, R. Thaler, T. Schwerd, K. Lahl, T. Sparwasser, R. Besch, H. Poeck, V. Hornung, G. Hartmann, S. Rothenfusser, C. Bourquin, and S. Endres. Immunostimulatory RNA blocks suppression by regulatory T cells. J. Immunol. 184:939-946. - PubMed
-
- Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. - PubMed
-
- Belkaid, Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7:875-888. - PubMed
-
- Biagi, E., I. Di Biaso, V. Leoni, G. Gaipa, V. Rossi, C. Bugarin, G. Renoldi, M. Parma, A. Balduzzi, P. Perseghin, and A. Biondi. 2007. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+ CD25+ GITR+ Foxp3+ CD62L+ functional regulatory T cells in patients with graft-versus-host disease. Transplantation 84:31-39. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

