Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling
- PMID: 20686047
- PMCID: PMC2950581
- DOI: 10.1128/JVI.00949-10
Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling
Abstract
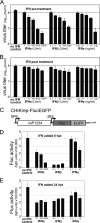
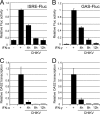
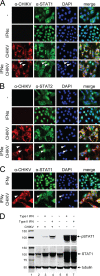
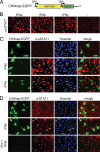
Chikungunya virus (CHIKV) is an emerging human pathogen transmitted by mosquitoes. Like that of other alphaviruses, CHIKV replication causes general host shutoff, leading to severe cytopathicity in mammalian cells, and inhibits the ability of infected cells to respond to interferon (IFN). Recent research, however, suggests that alphaviruses may have additional mechanisms to circumvent the host's antiviral IFN response. Here we show that CHIKV replication is resistant to inhibition by interferon once RNA replication has been established and that CHIKV actively suppresses the antiviral IFN response by preventing IFN-induced gene expression. Both CHIKV infection and CHIKV replicon RNA replication efficiently blocked STAT1 phosphorylation and/or nuclear translocation in mammalian cells induced by either type I or type II IFN. Expression of individual CHIKV nonstructural proteins (nsPs) showed that nsP2 was a potent inhibitor of IFN-induced JAK-STAT signaling. In addition, mutations in CHIKV-nsP2 (P718S) and Sindbis virus (SINV)-nsP2 (P726S) that render alphavirus replicons noncytopathic significantly reduced JAK-STAT inhibition. This host shutoff-independent inhibition of IFN signaling by CHIKV is likely to have an important role in viral pathogenesis.
Figures
Similar articles
-
Novel Mutations in nsP2 Abolish Chikungunya Virus-Induced Transcriptional Shutoff and Make the Virus Less Cytopathic without Affecting Its Replication Rates.J Virol. 2019 Feb 5;93(4):e02062-18. doi: 10.1128/JVI.02062-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30487275 Free PMC article.
-
The Methyltransferase-Like Domain of Chikungunya Virus nsP2 Inhibits the Interferon Response by Promoting the Nuclear Export of STAT1.J Virol. 2018 Aug 16;92(17):e01008-18. doi: 10.1128/JVI.01008-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925658 Free PMC article.
-
The C-terminal domain of chikungunya virus nsP2 independently governs viral RNA replication, cytopathicity, and inhibition of interferon signaling.J Virol. 2013 Sep;87(18):10394-400. doi: 10.1128/JVI.00884-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864632 Free PMC article.
-
Interferon-induced restriction of Chikungunya virus infection.Antiviral Res. 2023 Feb;210:105487. doi: 10.1016/j.antiviral.2022.105487. Epub 2022 Dec 22. Antiviral Res. 2023. PMID: 36657882 Review.
-
New Insights into Chikungunya Virus Infection and Pathogenesis.Annu Rev Virol. 2021 Sep 29;8(1):327-347. doi: 10.1146/annurev-virology-091919-102021. Epub 2021 Jul 13. Annu Rev Virol. 2021. PMID: 34255544 Review.
Cited by
-
Chikungunya virus nsP3 blocks stress granule assembly by recruitment of G3BP into cytoplasmic foci.J Virol. 2012 Oct;86(19):10873-9. doi: 10.1128/JVI.01506-12. Epub 2012 Jul 25. J Virol. 2012. PMID: 22837213 Free PMC article.
-
3Cpro of FMDV inhibits type II interferon-stimulated JAK-STAT signaling pathway by blocking STAT1 nuclear translocation.Virol Sin. 2023 Jun;38(3):387-397. doi: 10.1016/j.virs.2023.03.003. Epub 2023 Mar 14. Virol Sin. 2023. PMID: 36921803 Free PMC article.
-
Comparative Characterization of the Sindbis Virus Proteome from Mammalian and Invertebrate Hosts Identifies nsP2 as a Component of the Virion and Sorting Nexin 5 as a Significant Host Factor for Alphavirus Replication.J Virol. 2018 Jun 29;92(14):e00694-18. doi: 10.1128/JVI.00694-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743363 Free PMC article.
-
Chikungunya nsP2 protease is not a papain-like cysteine protease and the catalytic dyad cysteine is interchangeable with a proximal serine.Sci Rep. 2015 Nov 24;5:17125. doi: 10.1038/srep17125. Sci Rep. 2015. PMID: 26597768 Free PMC article.
-
Selective Inhibitor of Nuclear Export (SINE) Compounds Alter New World Alphavirus Capsid Localization and Reduce Viral Replication in Mammalian Cells.PLoS Negl Trop Dis. 2016 Nov 30;10(11):e0005122. doi: 10.1371/journal.pntd.0005122. eCollection 2016 Nov. PLoS Negl Trop Dis. 2016. PMID: 27902702 Free PMC article.
References
-
- Couderc, T., F. Chretien, C. Schilte, O. Disson, M. Brigitte, F. Guivel-Benhassine, Y. Touret, G. Barau, N. Cayet, I. Schuffenecker, P. Despres, F. Arenzana-Seisdedos, A. Michault, M. L. Albert, and M. Lecuit. 2008. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 4:e29. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

