Protein kinase C-{delta} regulates the subcellular localization of Shc in H2O2-treated cardiomyocytes
- PMID: 20686066
- PMCID: PMC2957271
- DOI: 10.1152/ajpcell.00170.2010
Protein kinase C-{delta} regulates the subcellular localization of Shc in H2O2-treated cardiomyocytes
Abstract
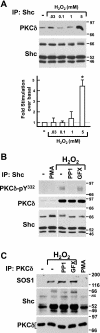
Protein kinase C-δ (PKCδ) exerts important cardiac actions as a lipid-regulated kinase. There is limited evidence that PKCδ also might exert an additional kinase-independent action as a regulator of the subcellular compartmentalization of binding partners such as Shc (Src homologous and collagen), a family of adapter proteins that play key roles in growth regulation and oxidative stress responses. This study shows that native PKCδ forms complexes with endogenous Shc proteins in H(2)O(2)-treated cardiomyocytes; H(2)O(2) treatment also leads to the accumulation of PKCδ and Shc in a detergent-insoluble cytoskeletal fraction and in mitochondria. H(2)O(2)-dependent recruitment of Shc isoforms to cytoskeletal and mitochondrial fractions is amplified by wild-type-PKCδ overexpression, consistent with the notion that PKCδ acts as a signal-regulated scaffold to anchor Shc in specific subcellular compartments. However, overexpression studies with kinase-dead (KD)-PKCδ-K376R (an ATP-binding mutant of PKCδ that lacks catalytic activity) are less informative, since KD-PKCδ-K376R aberrantly localizes as a constitutively tyrosine-phosphorylated enzyme to detergent-insoluble and mitochondrial fractions of resting cardiomyocytes; relatively little KD-PKCδ-K376R remains in the cytosolic fraction. The aberrant localization and tyrosine phosphorylation patterns for KD-PKCδ-K376R do not phenocopy the properties of native PKCδ, even in cells chronically treated with GF109203X to inhibit PKCδ activity. Hence, while KD-PKCδ-K376R overexpression increases Shc localization to the detergent-insoluble and mitochondrial fractions, the significance of these results is uncertain. Our studies suggest that experiments using KD-PKCδ-K376R overexpression as a strategy to competitively inhibit the kinase-dependent actions of native PKCδ or to expose the kinase-independent scaffolding functions of PKCδ should be interpreted with caution.
Figures





Comment in
-
Kinase activity-independent anchoring function of protein kinase C-{delta}. Focus on "Protein kinase C-{delta} regulates the subcellular localization of Shc in H2O2-treated cardiomyocytes".Am J Physiol Cell Physiol. 2010 Oct;299(4):C733-5. doi: 10.1152/ajpcell.00301.2010. Epub 2010 Aug 4. Am J Physiol Cell Physiol. 2010. PMID: 20686076 Free PMC article. No abstract available.
Similar articles
-
Kinase activity-independent anchoring function of protein kinase C-{delta}. Focus on "Protein kinase C-{delta} regulates the subcellular localization of Shc in H2O2-treated cardiomyocytes".Am J Physiol Cell Physiol. 2010 Oct;299(4):C733-5. doi: 10.1152/ajpcell.00301.2010. Epub 2010 Aug 4. Am J Physiol Cell Physiol. 2010. PMID: 20686076 Free PMC article. No abstract available.
-
p66Shc links alpha1-adrenergic receptors to a reactive oxygen species-dependent AKT-FOXO3A phosphorylation pathway in cardiomyocytes.Circ Res. 2009 Mar 13;104(5):660-9. doi: 10.1161/CIRCRESAHA.108.186288. Epub 2009 Jan 22. Circ Res. 2009. PMID: 19168439 Free PMC article.
-
Epidermal growth factor receptor phosphorylates protein kinase C {delta} at Tyr332 to form a trimeric complex with p66Shc in the H2O2-stimulated cells.J Biochem. 2008 Jan;143(1):31-8. doi: 10.1093/jb/mvm190. Epub 2007 Oct 23. J Biochem. 2008. PMID: 17956904
-
Distinctive activation mechanisms and functions for protein kinase Cdelta.Biochem J. 2004 Dec 15;384(Pt 3):449-59. doi: 10.1042/BJ20040704. Biochem J. 2004. PMID: 15491280 Free PMC article. Review.
-
The mitochondrial PKCδ/retinol signal complex exerts real-time control on energy homeostasis.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Nov;1865(11):158614. doi: 10.1016/j.bbalip.2020.158614. Epub 2020 Jan 10. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31927141 Free PMC article. Review.
Cited by
-
Sepsis-induced cardiac mitochondrial dysfunction involves altered mitochondrial-localization of tyrosine kinase Src and tyrosine phosphatase SHP2.PLoS One. 2012;7(8):e43424. doi: 10.1371/journal.pone.0043424. Epub 2012 Aug 27. PLoS One. 2012. PMID: 22952679 Free PMC article.
-
Pkcδ Activation is Involved in ROS-Mediated Mitochondrial Dysfunction and Apoptosis in Cardiomyocytes Exposed to Advanced Glycation End Products (Ages).Aging Dis. 2018 Aug 1;9(4):647-663. doi: 10.14336/AD.2017.0924. eCollection 2018 Aug. Aging Dis. 2018. PMID: 30090653 Free PMC article.
-
Protein kinase Cδ differentially regulates cAMP-dependent translocation of NTCP and MRP2 to the plasma membrane.Am J Physiol Gastrointest Liver Physiol. 2012 Sep 1;303(5):G657-65. doi: 10.1152/ajpgi.00529.2011. Epub 2012 Jun 28. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22744337 Free PMC article.
-
Cardiac actions of protein kinase C isoforms.Physiology (Bethesda). 2012 Jun;27(3):130-9. doi: 10.1152/physiol.00009.2012. Physiology (Bethesda). 2012. PMID: 22689788 Free PMC article. Review.
-
Intramolecular conformational changes optimize protein kinase C signaling.Chem Biol. 2014 Apr 24;21(4):459-469. doi: 10.1016/j.chembiol.2014.02.008. Epub 2014 Mar 13. Chem Biol. 2014. PMID: 24631122 Free PMC article.
References
-
- Avkiran M, Rowland AJ, Cuello F, Haworth RS. Protein kinase D in the cardiovascular system: emerging roles in health and disease. Circ Res 102: 157–163, 2008 - PubMed
-
- Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCδ is a phosphotyrosine binding domain. Cell 121: 271–280, 2005 - PubMed
-
- Bonfini L, Migliaccio E, Pelicci G, Lanfrancone L, Pelicci PG. Not all Shc's roads lead to Ras. Trends Biochem Sci 21: 257–261, 1996 - PubMed
-
- Cameron AJ, Escribano C, Saurin AT, Kostelecky B, Parker PJ. PKC maturation is promoted by nucleotide pocket occupation independently of intrinsic kinase activity. Nat Struct Mol Biol 16: 624–630, 2009 - PubMed
-
- Cameron AJ, Procyk KJ, Leitges M, Parker PJ. PKCα protein but not kinase activity is critical for glioma cell proliferation and survival. Int J Cancer 123: 769–779, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

