Characterization of hTERT-immortalized osteoblast cell lines generated from wild-type and connexin43-null mouse calvaria
- PMID: 20686067
- PMCID: PMC2980299
- DOI: 10.1152/ajpcell.00544.2009
Characterization of hTERT-immortalized osteoblast cell lines generated from wild-type and connexin43-null mouse calvaria
Abstract
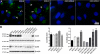
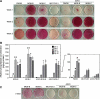
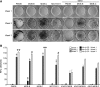
The gap junction protein connexin43 (Cx43) has been proposed to play key roles in bone differentiation and mineralization, but underlying cellular mechanisms are not totally understood. To further explore roles of Cx43 in these processes, we immortalized calvarial osteoblasts from wild-type and Cx43-null mice using human telomerase reverse transcriptase (hTERT). Osteoblastic (MOB) cell lines were generated from three individual wild-type and three individual Cx43-null mouse calvaria. Average population doubling times of the cell lines were higher than of the primary osteoblasts but did not greatly differ with regard to genotype. Modest to high level of Cx45 expression was detected in MOBs of both genotypes. Most of the cell lines expressed osteoblastic markers [Type I collagen, osteopontin, osteocalcin, parathyroid hormone/parathyroid hormone-related peptide receptor (PTH/PTHrP), periostin (OSF-2), osterix (Osx), runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP)], and mineralization was comparable to that of primary osteoblasts. Two MOB cell lines from each genotype with most robust maintenance of osteoblast lineage markers were analyzed in greater detail, revealing that the Cx43-null cell lines showed a significant delay in early differentiation (up to 9 days in culture). Matrix mineralization was markedly delayed in one of the Cx43-null lines and slightly delayed in the other. These findings comparing new and very stable wild-type and Cx43-null osteoblastic cell lines define a role for Cx43 in early differentiation and mineralization stages of osteoblasts and further support the concept that Cx43 plays important role in the cellular processes associated with skeleton function.
Figures






Similar articles
-
Proliferation, differentiation and apoptosis in connexin43-null osteoblasts.Cell Commun Adhes. 2001;8(4-6):367-71. doi: 10.3109/15419060109080755. Cell Commun Adhes. 2001. PMID: 12064620
-
Modulation of connexin43 alters expression of osteoblastic differentiation markers.Am J Physiol Cell Physiol. 2006 Apr;290(4):C1248-55. doi: 10.1152/ajpcell.00428.2005. Epub 2005 Nov 30. Am J Physiol Cell Physiol. 2006. PMID: 16319124
-
Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction.J Cell Biol. 2000 Nov 13;151(4):931-44. doi: 10.1083/jcb.151.4.931. J Cell Biol. 2000. PMID: 11076975 Free PMC article.
-
Molecular mechanisms of osteoblast/osteocyte regulation by connexin43.Calcif Tissue Int. 2014 Jan;94(1):55-67. doi: 10.1007/s00223-013-9742-6. Epub 2013 Jun 11. Calcif Tissue Int. 2014. PMID: 23754488 Free PMC article. Review.
-
Cell-cell communication in the osteoblast/osteocyte lineage.Arch Biochem Biophys. 2008 May 15;473(2):188-92. doi: 10.1016/j.abb.2008.04.005. Epub 2008 Apr 11. Arch Biochem Biophys. 2008. PMID: 18424255 Free PMC article. Review.
Cited by
-
Osteocyte calcium signals encode strain magnitude and loading frequency in vivo.Proc Natl Acad Sci U S A. 2017 Oct 31;114(44):11775-11780. doi: 10.1073/pnas.1707863114. Epub 2017 Oct 19. Proc Natl Acad Sci U S A. 2017. PMID: 29078317 Free PMC article.
-
Connexin43 and Runx2 Interact to Affect Cortical Bone Geometry, Skeletal Development, and Osteoblast and Osteoclast Function.J Bone Miner Res. 2017 Aug;32(8):1727-1738. doi: 10.1002/jbmr.3152. Epub 2017 May 22. J Bone Miner Res. 2017. PMID: 28419546 Free PMC article.
-
Isolation and characterization of a spontaneously immortalized multipotent mesenchymal cell line derived from mouse subcutaneous adipose tissue.Stem Cells Dev. 2013 Nov 1;22(21):2873-84. doi: 10.1089/scd.2012.0718. Epub 2013 Aug 9. Stem Cells Dev. 2013. PMID: 23777308 Free PMC article.
-
The transcriptional activity of osterix requires the recruitment of Sp1 to the osteocalcin proximal promoter.Bone. 2011 Oct;49(4):683-92. doi: 10.1016/j.bone.2011.07.027. Epub 2011 Jul 28. Bone. 2011. PMID: 21820092 Free PMC article.
-
Role of pannexin 1 channels in load-induced skeletal response.Ann N Y Acad Sci. 2019 Apr;1442(1):79-90. doi: 10.1111/nyas.13914. Epub 2018 Jun 28. Ann N Y Acad Sci. 2019. PMID: 29952014 Free PMC article.
References
-
- Aubin JE, Turksen K, Heersche JNM. Osteoblastic cell lineage. In: Cellular and Molecular Biology of Bone, edited by Noda M. San Diego, CA: Academic, 1993, p. 1–45
-
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science 279: 349–352, 1998 - PubMed
-
- Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, Boyan B, Boskey A. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int 72: 537–547, 2003 - PubMed
-
- Chen D, Chen H, Feng JQ, Windle JJ, Koop BA, Harris MA, Bonewald LF, Boyce BF, Wonzney JM, Mundy GR, Harris SE. Osteoblastic cell line derived from a transgenic mouse containing the osteocalcin promoter driving SV40 T-antigen. Mol Cell Differ 3: 193–212, 1995
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

