Cystic fibrosis transmembrane conductance regulator is involved in airway epithelial wound repair
- PMID: 20686068
- PMCID: PMC2980302
- DOI: 10.1152/ajpcell.00215.2010
Cystic fibrosis transmembrane conductance regulator is involved in airway epithelial wound repair
Abstract
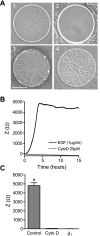
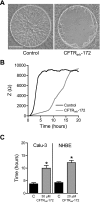
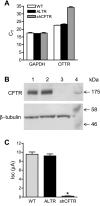
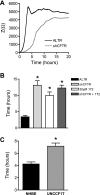
The role of cystic fibrosis (CF) transmembrane conductance regulator (CFTR) in airway epithelial wound repair was investigated using normal human bronchial epithelial (NHBE) cells and a human airway epithelial cell line (Calu-3) of serous gland origin. Measurements of wound repair were performed using continuous impedance sensing to determine the time course for wound closure. Control experiments showed that the increase in impedance corresponding to cell migration into the wound was blocked by treatment with the actin polymerization inhibitor, cytochalasin D. Time lapse imaging revealed that NHBE and Calu-3 cell wound closure was dependent on cell migration, and that movement occurred as a collective sheet of cells. Selective inhibition of CFTR activity with CFTR(inh)-172 or short hairpin RNA silencing of CFTR expression produced a significant delay in wound repair. The CF cell line UNCCF1T also exhibited significantly slower migration than comparable normal airway epithelial cells. Inhibition of CFTR-dependent anion transport by treatment with CFTR(inh)-172 slowed wound closure to the same extent as silencing CFTR protein expression, indicating that ion transport by CFTR plays a critical role in migration. Moreover, morphologic analysis of migrating cells revealed that CFTR inhibition or silencing significantly reduced lamellipodia protrusion. These findings support the conclusion that CFTR participates in airway epithelial wound repair by a mechanism involving anion transport that is coupled to the regulation of lamellipodia protrusion at the leading edge of the cell.
Figures

Comment in
-
CFTR channels and wound healing. Focus on "Cystic fibrosis transmembrane conductance regulator is involved in airway epithelial wound repair".Am J Physiol Cell Physiol. 2010 Nov;299(5):C888-90. doi: 10.1152/ajpcell.00313.2010. Epub 2010 Aug 11. Am J Physiol Cell Physiol. 2010. PMID: 20702686 No abstract available.
References
-
- Alexander RT, Grinstein S. Na+/H+ exchangers and the regulation of volume. Acta Physiol (Oxf) 187: 159–167, 2006 - PubMed
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253: 202–205, 1991 - PubMed
-
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23: 146–158, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

