Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption
- PMID: 20686069
- PMCID: PMC2980313
- DOI: 10.1152/ajpcell.00113.2010
Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption
Abstract
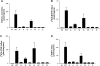
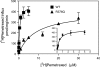
The proton-coupled folate transporter (PCFT-SLC46A1) is required for intestinal folate absorption and is mutated in the autosomal recessive disorder, hereditary folate malabsorption (HFM). This report characterizes properties and requirements of the R376 residue in PCFT function, including a R376Q mutant associated with HFM. Gln, Cys, and Ala substitutions resulted in markedly impaired transport of 5-formyltetrahydrofolate (5-FTHF) and 5-methyltetrahydrofolate (5-MTHF) due to an increase in K(m) and decrease in V(max) in HeLa R1-11 transfectants lacking endogenous folate transport function. In contrast, although the influx K(m) for pemetrexed was increased, transport was fully preserved at saturating concentrations and enhanced for the like-charged R376K- and R376H-PCFT. Pemetrexed and 5-FTHF influx mediated by R376Q-PCFT was markedly decreased at pH 5.5 compared with wild-type PCFT. However, while pemetrexed transport was substantially preserved at low pH (4.5-5.0), 5-FTHF transport remained very low. Electrophysiological studies in Xenopus oocytes demonstrated that 1) the R376Q mutant, like wild-type PCFT, transports protons in the absence of folate substrate, and in this respect has channel-like properties; and 2) the influx K(m) mediated by R376Q-PCFT is increased for 5-MTHF, 5-FTHF, and pemetrexed. The data suggest that mutation of the R376 residue to Gln impairs proton binding which, in turn, modulates the folate-binding pocket and depresses the rate of conformational alteration of the carrier, a change that appears to be, in part, substrate dependent.
Figures

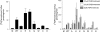
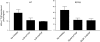
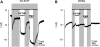

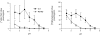
References
-
- Cario H, Bode H, Debatin KM, Opladen T, Schwarz K. Congenital null mutations of the FOLR1 gene: a progressive neurologic disease and its treatment. Neurology 73: 2127–2129, 2009 - PubMed
-
- Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 6: 404–417, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

