Expression profile and protein translation of TMEM16A in murine smooth muscle
- PMID: 20686072
- PMCID: PMC2980309
- DOI: 10.1152/ajpcell.00018.2010
Expression profile and protein translation of TMEM16A in murine smooth muscle
Abstract
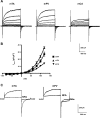
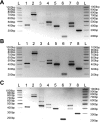
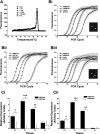
Recently, overexpression of the genes TMEM16A and TMEM16B has been shown to produce currents qualitatively similar to native Ca(2+)-activated Cl(-) currents (I(ClCa)) in vascular smooth muscle. However, there is no information about this new gene family in vascular smooth muscle, where Cl(-) channels are a major depolarizing mechanism. Qualitatively similar Cl(-) currents were evoked by a pipette solution containing 500 nM Ca(2+) in smooth muscle cells isolated from BALB/c mouse portal vein, thoracic aorta, and carotid artery. Quantitative PCR using SYBR Green chemistry and primers specific for transmembrane protein (TMEM) 16A or the closely related TMEM16B showed TMEM16A expression as follows: portal vein > thoracic aorta > carotid artery > brain. In addition, several alternatively spliced variant transcripts of TMEM16A were detected. In contrast, TMEM16B expression was very low in smooth muscle. Western blot analysis with different antibodies directed against TMEM16A revealed a number of products with a consistent band at ∼120 kDa, except portal vein, where an 80-kDa band predominated. TMEM16A protein was identified in the smooth muscle layers of 4-μm-thick slices of portal vein, thoracic aorta, and carotid artery. In isolated myocytes, fluorescence specific to a TMEM16A antibody was detected diffusely throughout the cytoplasm, as well as near the membrane. The same antibody used in Western blot analysis of lysates from vascular tissues also recognized an ∼147-kDa mouse TMEM16A-green fluorescent protein (GFP) fusion protein expressed in HEK 293 cells, which correlated to a similar band detected by a GFP antibody. Patch-clamp experiments revealed that I(ClCa) generated by transfection of TMEM16A-GFP in HEK 293 cells displayed remarkable similarities to I(ClCa) recorded in vascular myocytes, including slow kinetics, steep outward rectification, and a response similar to the pharmacological agent niflumic acid. This study shows that TMEM16A expression is robust in murine vascular smooth muscle cells, consolidating the view that this gene is a viable candidate for the native Ca(2+)-activated Cl(-) channel in this cell type.
Figures





Similar articles
-
TMEM16A channels generate Ca²⁺-activated Cl⁻ currents in cerebral artery smooth muscle cells.Am J Physiol Heart Circ Physiol. 2011 Nov;301(5):H1819-27. doi: 10.1152/ajpheart.00404.2011. Epub 2011 Aug 19. Am J Physiol Heart Circ Physiol. 2011. PMID: 21856902 Free PMC article.
-
The transmembrane protein 16A Ca(2+)-activated Cl- channel in airway smooth muscle contributes to airway hyperresponsiveness.Am J Respir Crit Care Med. 2013 Feb 15;187(4):374-81. doi: 10.1164/rccm.201207-1303OC. Epub 2012 Dec 13. Am J Respir Crit Care Med. 2013. PMID: 23239156 Free PMC article.
-
Increased TMEM16A-Mediated Ca2+-Activated Cl- Currents in Portal Vein Smooth Muscle Cells of Caveolin 1-Deficient Mice.Biol Pharm Bull. 2022 Nov 1;45(11):1692-1698. doi: 10.1248/bpb.b22-00514. Epub 2022 Aug 20. Biol Pharm Bull. 2022. PMID: 35989294
-
The role of Transmembrane Protein 16A (TMEM16A) in pulmonary hypertension.Cardiovasc Pathol. 2023 Jul-Aug;65:107525. doi: 10.1016/j.carpath.2023.107525. Epub 2023 Feb 11. Cardiovasc Pathol. 2023. PMID: 36781068 Review.
-
The TMEM16A channel as a potential therapeutic target in vascular disease.Curr Opin Nephrol Hypertens. 2024 Mar 1;33(2):161-169. doi: 10.1097/MNH.0000000000000967. Epub 2024 Jan 8. Curr Opin Nephrol Hypertens. 2024. PMID: 38193301 Free PMC article. Review.
Cited by
-
TMEM16 proteins: Ca2+‑activated chloride channels and phospholipid scramblases as potential drug targets (Review).Int J Mol Med. 2024 Oct;54(4):81. doi: 10.3892/ijmm.2024.5405. Epub 2024 Aug 2. Int J Mol Med. 2024. PMID: 39092585 Free PMC article. Review.
-
Polymodal Control of TMEM16x Channels and Scramblases.Int J Mol Sci. 2022 Jan 29;23(3):1580. doi: 10.3390/ijms23031580. Int J Mol Sci. 2022. PMID: 35163502 Free PMC article. Review.
-
Anion permeation in calcium-activated chloride channels formed by TMEM16A from Xenopus tropicalis.Pflugers Arch. 2014 Sep;466(9):1769-77. doi: 10.1007/s00424-013-1415-9. Epub 2013 Dec 19. Pflugers Arch. 2014. PMID: 24352628
-
TMEM16A knockdown abrogates two different Ca(2+)-activated Cl (-) currents and contractility of smooth muscle in rat mesenteric small arteries.Pflugers Arch. 2014 Jul;466(7):1391-409. doi: 10.1007/s00424-013-1382-1. Epub 2013 Oct 27. Pflugers Arch. 2014. PMID: 24162234 Free PMC article.
-
Drug Repurposing: The Anthelmintics Niclosamide and Nitazoxanide Are Potent TMEM16A Antagonists That Fully Bronchodilate Airways.Front Pharmacol. 2019 Feb 14;10:51. doi: 10.3389/fphar.2019.00051. eCollection 2019. Front Pharmacol. 2019. PMID: 30837866 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

