PCR-based pooling of dried blood spots for detection of malaria parasites: optimization and application to a cohort of Ugandan children
- PMID: 20686079
- PMCID: PMC2953134
- DOI: 10.1128/JCM.00522-10
PCR-based pooling of dried blood spots for detection of malaria parasites: optimization and application to a cohort of Ugandan children
Abstract
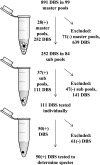
Sensitive, high-throughput methods to detect malaria parasites in low-transmission settings are needed. PCR-based pooling strategies may offer a solution. We first used laboratory-prepared samples to compare 2 DNA extraction and 4 PCR detection methods across a range of pool sizes and parasite densities. Pooled Chelex extraction of DNA, followed by nested PCR of cytochrome b, was the optimal strategy, allowing reliable detection of a single low-parasitemic sample (100 parasites/μl) in pool sizes up to 50. This PCR-based pooling strategy was then compared with microscopy using 891 dried blood spots from a cohort of 77 Ugandan children followed for 2 years in an urban setting of low endemicity. Among 419 febrile episodes, 35 cases of malaria were detected using the PCR-based pooling strategy and 40 cases using microscopy. All five cases of malaria not detected by PCR were from samples stored for >2 years with parasitemia of <6,000/μl, highlighting the issue of possible DNA degradation with long-term storage of samples. Among 472 samples collected from asymptomatic children as part of routine surveillance, 15 (3.2%) were positive by PCR-based pooling compared to 4 (0.8%) by microscopy (P = 0.01). Thus, this PCR-based pooling strategy for detection of malaria parasites using dried blood spots offers a sensitive and efficient approach for malaria surveillance in low-transmission settings, enabling improved detection of asymptomatic submicroscopic infections and dramatic savings in labor and costs.
Figures

Similar articles
-
Effective High-Throughput Blood Pooling Strategy before DNA Extraction for Detection of Malaria in Low-Transmission Settings.Korean J Parasitol. 2016 Jun;54(3):253-9. doi: 10.3347/kjp.2016.54.3.253. Epub 2016 Jun 30. Korean J Parasitol. 2016. PMID: 27417078 Free PMC article.
-
Comparison of methods for detecting asymptomatic malaria infections in the China-Myanmar border area.Malar J. 2017 Apr 20;16(1):159. doi: 10.1186/s12936-017-1813-0. Malar J. 2017. PMID: 28427455 Free PMC article.
-
Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women.Malar J. 2010 Oct 6;9:269. doi: 10.1186/1475-2875-9-269. Malar J. 2010. PMID: 20925928 Free PMC article.
-
Pre-amplification methods for tracking low-grade Plasmodium falciparum populations during scaled-up interventions in Southern Zambia.Malar J. 2014 Mar 12;13:89. doi: 10.1186/1475-2875-13-89. Malar J. 2014. PMID: 24618119 Free PMC article.
-
Automated haematology analysis to diagnose malaria.Malar J. 2010 Nov 30;9:346. doi: 10.1186/1475-2875-9-346. Malar J. 2010. PMID: 21118557 Free PMC article. Review.
Cited by
-
Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets.PLoS Med. 2015 Mar 3;12(3):e1001788. doi: 10.1371/journal.pmed.1001788. eCollection 2015 Mar. PLoS Med. 2015. PMID: 25734259 Free PMC article.
-
Safety of a single low-dose of primaquine in addition to standard artemether-lumefantrine regimen for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania.Malar J. 2016 Jun 10;15:316. doi: 10.1186/s12936-016-1341-3. Malar J. 2016. PMID: 27287612 Free PMC article. Clinical Trial.
-
Template copy number and the sensitivity of quantitative PCR for Plasmodium falciparum in asymptomatic individuals.Malar J. 2020 Aug 18;19(1):295. doi: 10.1186/s12936-020-03365-8. Malar J. 2020. PMID: 32811534 Free PMC article.
-
Tenfold difference in DNA recovery rate: systematic comparison of whole blood vs. dried blood spot sample collection for malaria molecular surveillance.Malar J. 2022 Mar 15;21(1):88. doi: 10.1186/s12936-022-04122-9. Malar J. 2022. PMID: 35292038 Free PMC article.
-
Characterization of Plasmodium Lactate Dehydrogenase and Histidine-Rich Protein 2 Clearance Patterns via Rapid On-Bead Detection from a Single Dried Blood Spot.Am J Trop Med Hyg. 2018 May;98(5):1389-1396. doi: 10.4269/ajtmh.17-0996. Epub 2018 Mar 15. Am J Trop Med Hyg. 2018. PMID: 29557342 Free PMC article.
References
-
- Boudin, C., M. Olivier, J. F. Molez, J. P. Chiron, and P. Ambroise-Thomas. 1993. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am. J. Trop. Med. Hyg. 48:700-706. - PubMed
-
- Busch, M. P., D. J. Wright, B. Custer, L. H. Tobler, S. L. Stramer, S. H. Kleinman, H. E. Prince, C. Bianco, G. Foster, L. R. Petersen, G. Nemo, and S. A. Glynn. 2006. West Nile virus infections projected from blood donor screening data, United States, 2003. Emerg. Infect. Dis. 12:395-402. - PMC - PubMed
-
- Clark, T. D., D. Njama-Meya, B. Nzarubara, C. Maiteki-Sebuguzi, B. Greenhouse, S. G. Staedke, M. R. Kamya, G. Dorsey, and P. J. Rosenthal. 2010. Incidence of malaria and efficacy of combination antimalarial therapies over 4 years in an urban cohort of Ugandan children. PLoS One 5:e11759. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

