Ferroportin and iron regulation in breast cancer progression and prognosis
- PMID: 20686179
- PMCID: PMC3734848
- DOI: 10.1126/scitranslmed.3001127
Ferroportin and iron regulation in breast cancer progression and prognosis
Erratum in
- Sci Transl Med. 2010 Aug 25;2(46):46er1
Abstract
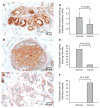
Ferroportin and hepcidin are critical proteins for the regulation of systemic iron homeostasis. Ferroportin is the only known mechanism for export of intracellular non-heme-associated iron; its stability is regulated by the hormone hepcidin. Although ferroportin profoundly affects concentrations of intracellular iron in tissues important for systemic iron absorption and trafficking, ferroportin concentrations in breast cancer and their influence on growth and prognosis have not been examined. We demonstrate here that both ferroportin and hepcidin are expressed in cultured human breast epithelial cells and that hepcidin regulates ferroportin in these cells. Further, ferroportin protein is substantially reduced in breast cancer cells compared to nonmalignant breast epithelial cells; ferroportin protein abundance correlates with metabolically available iron. Ferroportin protein is also present in normal human mammary tissue and markedly decreased in breast cancer tissue, with the highest degree of anaplasia associated with lowest ferroportin expression. Transfection of breast cancer cells with ferroportin significantly reduces their growth after orthotopic implantation in the mouse mammary fat pad. Gene expression profiles in breast cancers from >800 women reveal that decreased ferroportin gene expression is associated with a significant reduction in metastasis-free and disease-specific survival that is independent of other breast cancer risk factors. High ferroportin and low hepcidin gene expression identifies an extremely favorable cohort of breast cancer patients who have a 10-year survival of >90%. Ferroportin is a pivotal protein in breast biology and a strong and independent predictor of prognosis in breast cancer.
Figures





References
-
- Thelander L, Gräslund A, Thelander M. Continual presence of oxygen and iron required for mammalian ribonucleotide reduction: Possible regulation mechanism. Biochem Biophys Res Commun. 1983;110:859–865. - PubMed
-
- Buss JL, Torti FM, Torti SV. The role of iron chelation in cancer therapy. Curr Med Chem. 2003;10:1021–1034. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

