A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease
- PMID: 20686425
- PMCID: PMC2950888
- DOI: 10.1097/MPH.0b013e3181e793f9
A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease
Abstract
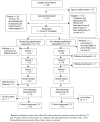
The study had 2 aims---to determine the efficacy of a family-based cognitive-behavioral pain management intervention for adolescents with sickle cell disease (SCD) in (1) reducing pain and improving health-related variables and (2) improving psychosocial outcomes. Each adolescent and a family support person were randomly assigned to receive a brief pain intervention (PAIN) (n=27) or a disease education attention control intervention (DISEASE ED) (n=26) delivered at home. Assessment of primary pain and health-related variables (health service use, pain coping, pain-related hindrance of goals) and secondary psychosocial outcomes (disease knowledge, disease self-efficacy, and family communication) occurred at baseline (before randomization), postintervention, and 1-year follow-up. Change on outcomes did not differ significantly by group at either time point. When groups were combined in exploratory analyses, there was evidence of small to medium effects of intervention on health-related and psychosocial variables. Efforts to address barriers to participation and improve feasibility of psychosocial interventions for pediatric SCD are critical to advancing development of effective treatments for pain. Sample size was insufficient to adequately test efficacy, and analyses did not support this focused cognitive-behavioral pain management intervention in this sample of adolescents with SCD. Exploratory analyses suggest that comprehensive interventions, that address a broad range of skills related to disease management and adolescent health concerns, may be more effective in supporting teens during healthcare transition.
Figures
References
-
- National Institutes of Health, National Heart, Lung, and Blood Institute, Division of Blood Diseases and Resources. The Management of Sickle Cell Disease. National Institutes of Health; 2002.
-
- Lemanek KL, Ranalli MA, Green K, et al. In: Handbook of pediatric psychology. 3. Roberts MC, editor. New York: Guilford; 2003. pp. 321–341.
-
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease: Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. - PubMed
-
- Palermo TM, Schwartz L, Drotar D, et al. Parental report of health-related quality of life in children with sickle cell disease. J Behav Med. 2002;25:269–283. - PubMed
-
- Telfair J, Ehiri JE, Loosier PS, et al. Transition to adult care for adolescents with sickle cell disease: Results of a national survey. Int J Adolesc Med Health. 2004;16:47–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical