Ventral striatal noradrenergic mechanisms contribute to sensorimotor gating deficits induced by amphetamine
- PMID: 20686455
- PMCID: PMC2955791
- DOI: 10.1038/npp.2010.106
Ventral striatal noradrenergic mechanisms contribute to sensorimotor gating deficits induced by amphetamine
Abstract
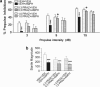
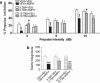
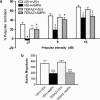
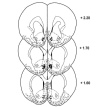
The psychotomimetic drug D-amphetamine (AMPH), disrupts prepulse inhibition (PPI) of the startle response, an operational measure of sensorimotor gating that is deficient in schizophrenia patients. Historically, this effect has been attributed to dopaminergic substrates; however, AMPH also increases norepinephrine (NE) levels, and enhancement of central NE transmission has been shown recently to disrupt PPI. This study examined the extent to which NE might participate in AMPH-induced disruptions of PPI and increases in locomotor activity, another classic behavioral effect of AMPH, by determining whether antagonism of postsynaptic NE receptors blocked these effects. Separate groups of male Sprague-Dawley rats received either the α1 receptor antagonist, prazosin (0, 0.3, 1 mg/kg), or the β receptor antagonist timolol (0, 3, 10 mg/kg) before administration of AMPH (0 or 1 mg/kg) before testing for PPI or locomotor activity. As an initial exploration of the anatomical substrates underlying possible α1 receptor-mediated effects on AMPH-induced PPI deficits, the α1 receptor antagonist terazosin (0 or 40 μg/0.5 μl) was microinfused into the nucleus accumbens shell (NAccSh) in conjunction with systemic AMPH administration before startle testing in a separate experiment. Prazosin, but not timolol, blocked AMPH-induced hyperactivity; both drugs reversed AMPH-induced PPI deficits without altering baseline startle responses. Interestingly, AMPH-induced PPI deficits also were partially blocked by terazosin in NAccSh. Thus, behavioral sequelae of AMPH (PPI disruption and hyperactivity) may be mediated in part by NE receptors, with α1 receptors in NAccSh possibly having an important role in the sensorimotor gating deficits induced by this psychotomimetic drug.
Figures
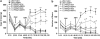
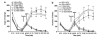
References
-
- Alsene KM, Carasso BS, Connors EE, Bakshi VP. Disruption of prepulse inhibition after stimulation of central but not peripheral alpha-1 adrenergic receptors. Neuropsychopharmacology. 2006;31:2150–2161. - PubMed
-
- Alsene KM, Ramaker MJ, Bakshi VP. Effects of noradrenergic receptor stimulation in the nucleus accumbens and anterior medial prefrontal cortex on prepulse inhibition. Soc Neurosci Abstr. 2007;33:499–527.
-
- Arnsten AF, Scahill L, Findling RL. Alpha2-adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol. 2007;17:393–406. - PubMed
-
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. - PubMed
-
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci. 2004;20:3073–3084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

