Identity of epibiotic bacteria on symbiontid euglenozoans in O2-depleted marine sediments: evidence for symbiont and host co-evolution
- PMID: 20686514
- PMCID: PMC3105687
- DOI: 10.1038/ismej.2010.121
Identity of epibiotic bacteria on symbiontid euglenozoans in O2-depleted marine sediments: evidence for symbiont and host co-evolution
Abstract
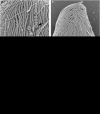
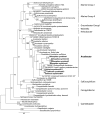
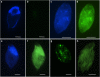
A distinct subgroup of euglenozoans, referred to as the 'Symbiontida,' has been described from oxygen-depleted and sulfidic marine environments. By definition, all members of this group carry epibionts that are intimately associated with underlying mitochondrion-derived organelles beneath the surface of the hosts. We have used molecular phylogenetic and ultrastructural evidence to identify the rod-shaped epibionts of the two members of this group, Calkinsia aureus and B.bacati, hand-picked from the sediments of two separate oxygen-depleted, sulfidic environments. We identify their epibionts as closely related sulfur or sulfide-oxidizing members of the epsilon proteobacteria. The epsilon proteobacteria generally have a significant role in deep-sea habitats as primary colonizers, primary producers and/or in symbiotic associations. The epibionts likely fulfill a role in detoxifying the immediate surrounding environment for these two different hosts. The nearly identical rod-shaped epibionts on these two symbiontid hosts provides evidence for a co-evolutionary history between these two sets of partners. This hypothesis is supported by congruent tree topologies inferred from 18S and 16S rDNA from the hosts and bacterial epibionts, respectively. The eukaryotic hosts likely serve as a motile substrate that delivers the epibionts to the ideal locations with respect to the oxic/anoxic interface, whereby their growth rates can be maximized, perhaps also allowing the host to cultivate a food source. Because symbiontid isolates and additional small subunit rDNA gene sequences from this clade have now been recovered from many locations worldwide, the Symbiontida are likely more widespread and diverse than presently known.
Figures


Similar articles
-
Euglenozoa: taxonomy, diversity and ecology, symbioses and viruses.Open Biol. 2021 Mar;11(3):200407. doi: 10.1098/rsob.200407. Epub 2021 Mar 10. Open Biol. 2021. PMID: 33715388 Free PMC article. Review.
-
Ultrastructure and molecular phylogenetic position of a novel euglenozoan with extrusive episymbiotic bacteria: Bihospites bacati n. gen. et sp. (Symbiontida).BMC Microbiol. 2010 May 19;10:145. doi: 10.1186/1471-2180-10-145. BMC Microbiol. 2010. PMID: 20482870 Free PMC article.
-
Ultrastructure and molecular phylogeny of Calkinsia aureus: cellular identity of a novel clade of deep-sea euglenozoans with epibiotic bacteria.BMC Microbiol. 2009 Jan 27;9:16. doi: 10.1186/1471-2180-9-16. BMC Microbiol. 2009. PMID: 19173734 Free PMC article.
-
Ectosymbiotic bacteria at the origin of magnetoreception in a marine protist.Nat Microbiol. 2019 Jul;4(7):1088-1095. doi: 10.1038/s41564-019-0432-7. Epub 2019 Apr 29. Nat Microbiol. 2019. PMID: 31036911 Free PMC article.
-
The versatile epsilon-proteobacteria: key players in sulphidic habitats.Nat Rev Microbiol. 2006 Jun;4(6):458-68. doi: 10.1038/nrmicro1414. Nat Rev Microbiol. 2006. PMID: 16652138 Review.
Cited by
-
Euglenozoa: taxonomy, diversity and ecology, symbioses and viruses.Open Biol. 2021 Mar;11(3):200407. doi: 10.1098/rsob.200407. Epub 2021 Mar 10. Open Biol. 2021. PMID: 33715388 Free PMC article. Review.
-
Diversity and distribution of eukaryotic microbes in and around a brine pool adjacent to the Thuwal cold seeps in the Red Sea.Front Microbiol. 2014 Feb 4;5:37. doi: 10.3389/fmicb.2014.00037. eCollection 2014. Front Microbiol. 2014. PMID: 24575081 Free PMC article.
-
Microbial eukaryote diversity in the marine oxygen minimum zone off northern Chile.Front Microbiol. 2014 Oct 28;5:543. doi: 10.3389/fmicb.2014.00543. eCollection 2014. Front Microbiol. 2014. PMID: 25389417 Free PMC article.
-
First Description of Sulphur-Oxidizing Bacterial Symbiosis in a Cnidarian (Medusozoa) Living in Sulphidic Shallow-Water Environments.PLoS One. 2015 May 26;10(5):e0127625. doi: 10.1371/journal.pone.0127625. eCollection 2015. PLoS One. 2015. PMID: 26011278 Free PMC article.
-
Distribution Patterns of Microeukaryotic Community Between Sediment and Water of the Yellow River Estuary.Curr Microbiol. 2020 Aug;77(8):1496-1505. doi: 10.1007/s00284-020-01958-9. Epub 2020 Apr 1. Curr Microbiol. 2020. PMID: 32239287
References
-
- Atkins MS, Hanna MA, Kupetsky EA, Saito MA, Taylor CD, Wirsen CO. Tolerance of flagellated protists to high sulfide and metal concentrations potentially encountered at deep-sea hydrothermal vents. Mar Ecol Prog Ser. 2002;226:63–75.
-
- Barbera MJ, Ruiz-Trillo I, Leigh J, Hug LA, Roger AJ.2007The Diversity of Mitochondrion-Related Organelles Amongst Eukaryotic Microbes In Origin of mitochondria and hydrogenosomesMartin WF, Muller, M Eds., Springer, Heidelberg: Germany; 239–275.
-
- Barry JP, Greene HG, Orange DL, Baxter CH, Robinson BH, Kochevar RE, et al. Biologic and geologic characteristics of cold seeps in Monterey Bay, California. Deep-Sea Res. 1996;43:1739–1762.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

