Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability
- PMID: 20686524
- PMCID: PMC4007820
- DOI: 10.1038/aps.2010.98
Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability
Abstract
Aim: To develop a novel vehicle based on cubosomes as an ophthalmic drug delivery system for flurbiprofen (FB) to reduce ocular irritancy and improve bioavailability.
Methods: FB-loaded cubosomes were prepared using hot and high-pressure homogenization. Cubosomes were then characterized by particle size, zeta potential, encapsulation efficiency, particle morphology, inner cubic structure and in vitro release. Corneal permeation was evaluated using modified Franz-type cells. Ocular irritation was then evaluated using both the Draize method and histological examination. The ocular pharmacokinetics of FB was determined using microdialysis.
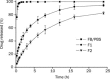
Results: The particle size of each cubosome formulation was about 150 nm. A bicontinuous cubic phase of cubic P-type was determined using cryo-transmission electron microscopy (cryo-TEM) observation and small angle X-ray scattering (SAXS) analysis. In vitro corneal permeation study revealed that FB formulated in cubosomes exhibited 2.5-fold (F1) and 2.0-fold (F2) increase in P(app) compared with FB PBS. In the ocular irritation test, irritation scores for each group were less than 2, indicating that all formulations exhibited excellent ocular tolerance. Histological examination revealed that neither the structure nor the integrity of the cornea was visibly affected after incubation with FB cubosomes. The AUC of FB administered as FB cubosome F2 was 486.36+/-38.93 ng.mL(-1).min.microg(-1), which was significantly higher than that of FB Na eye drops (P<0.01). Compared with FB Na eye drops, the T(max) of FB cubosome F2 was about 1.6-fold higher and the MRT was also significantly longer (P<0.001).
Conclusion: This novel low-irritant vehicle based on cubosomes might be a promising system for effective ocular drug delivery.
Figures






Similar articles
-
Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability.Int J Pharm. 2010 Aug 30;396(1-2):179-87. doi: 10.1016/j.ijpharm.2010.06.015. Epub 2010 Jun 15. Int J Pharm. 2010. PMID: 20558263
-
Novel NSAIDs ophthalmic formulation: flurbiprofen axetil emulsion with low irritancy and improved anti-inflammation effect.Int J Pharm. 2011 Jun 30;412(1-2):115-22. doi: 10.1016/j.ijpharm.2011.03.041. Epub 2011 Apr 2. Int J Pharm. 2011. PMID: 21440613
-
[Ion-sensitive nanoemulsion-in situ gel system for ophthalmic delivery of flurbiprofen axetil].Yao Xue Xue Bao. 2010 Jan;45(1):120-5. Yao Xue Xue Bao. 2010. PMID: 21351461 Chinese.
-
Drug Delivery for Ocular Allergy: Current Formulation Design Strategies and Future Perspectives.Curr Pharm Des. 2023;29(33):2626-2639. doi: 10.2174/0113816128275375231030115828. Curr Pharm Des. 2023. PMID: 37936454 Review.
-
Vesicular approach of cubosomes, its components, preparation techniques, evaluation and their appraisal for targeting cancer cells.J Liposome Res. 2024 Jun;34(2):368-384. doi: 10.1080/08982104.2023.2272643. Epub 2023 Oct 24. J Liposome Res. 2024. PMID: 37873797 Review.
Cited by
-
Development and Optimization of Hyaluronic Acid-Poloxamer In-Situ Gel Loaded with Voriconazole Cubosomes for Enhancement of Activity against Ocular Fungal Infection.Gels. 2022 Apr 14;8(4):241. doi: 10.3390/gels8040241. Gels. 2022. PMID: 35448142 Free PMC article.
-
Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells.Molecules. 2019 Aug 22;24(17):3058. doi: 10.3390/molecules24173058. Molecules. 2019. PMID: 31443533 Free PMC article.
-
Nanostructured Cubosomes in a Thermoresponsive Depot System: An Alternative Approach for the Controlled Delivery of Docetaxel.AAPS PharmSciTech. 2016 Apr;17(2):436-45. doi: 10.1208/s12249-015-0369-y. Epub 2015 Jul 25. AAPS PharmSciTech. 2016. PMID: 26208439 Free PMC article.
-
Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine.RSC Adv. 2019 Feb 21;9(11):6287-6298. doi: 10.1039/c8ra10302j. eCollection 2019 Feb 18. RSC Adv. 2019. PMID: 35517286 Free PMC article.
-
Enhanced Skin Permeation and Controlled Release of β-Sitosterol Using Cubosomes Encrusted with Dissolving Microneedles for the Management of Alopecia.Pharmaceuticals (Basel). 2023 Apr 8;16(4):563. doi: 10.3390/ph16040563. Pharmaceuticals (Basel). 2023. PMID: 37111320 Free PMC article.
References
-
- Waterbury L, Kunysz EA, Bewerman R. Effect of steroidal and non steroidal anti-inflammatory agents on corneal wound healing. J Ocul Pharmacol. 1987;3:43–54. - PubMed
-
- Schalnus R. Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica. 2003;217:89–98. - PubMed
-
- Joseph TM. A critical look at ocular allergy drugs. Am Fam Physician. 1996;53:2637–42. - PubMed
-
- Stroobants A, Fabre K, Maudgal PC. Effect of non-steroidal antiinflammatory drugs (NSAID) on the rabbit corneal epithelium studied by scanning electron microscopy. Bull Soc Belge Ophtalmol. 2000;276:73–81. - PubMed
-
- Araújo J, Vega E, Lopes C, Egea MA, Garcia ML, Souto EB. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloid Surfaces B. 2009;72:48–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

