Branched tricarboxylic acid metabolism in Plasmodium falciparum
- PMID: 20686576
- PMCID: PMC2917841
- DOI: 10.1038/nature09301
Branched tricarboxylic acid metabolism in Plasmodium falciparum
Erratum in
- Nature. 2011 Jan 20;469(7330):432
Retraction in
-
Retraction: Branched tricarboxylic acid metabolism in Plasmodium falciparum.Nature. 2013 May 30;497(7451):652. doi: 10.1038/nature12164. Epub 2013 Apr 24. Nature. 2013. PMID: 23615611 Free PMC article. No abstract available.
Abstract
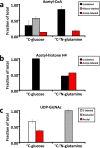
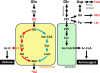
A central hub of carbon metabolism is the tricarboxylic acid cycle, which serves to connect the processes of glycolysis, gluconeogenesis, respiration, amino acid synthesis and other biosynthetic pathways. The protozoan intracellular malaria parasites (Plasmodium spp.), however, have long been suspected of possessing a significantly streamlined carbon metabolic network in which tricarboxylic acid metabolism plays a minor role. Blood-stage Plasmodium parasites rely almost entirely on glucose fermentation for energy and consume minimal amounts of oxygen, yet the parasite genome encodes all of the enzymes necessary for a complete tricarboxylic acid cycle. Here, by tracing (13)C-labelled compounds using mass spectrometry we show that tricarboxylic acid metabolism in the human malaria parasite Plasmodium falciparum is largely disconnected from glycolysis and is organized along a fundamentally different architecture from the canonical textbook pathway. We find that this pathway is not cyclic, but rather is a branched structure in which the major carbon sources are the amino acids glutamate and glutamine. As a consequence of this branched architecture, several reactions must run in the reverse of the standard direction, thereby generating two-carbon units in the form of acetyl-coenzyme A. We further show that glutamine-derived acetyl-coenzyme A is used for histone acetylation, whereas glucose-derived acetyl-coenzyme A is used to acetylate amino sugars. Thus, the parasite has evolved two independent production mechanisms for acetyl-coenzyme A with different biological functions. These results significantly clarify our understanding of the Plasmodium metabolic network and highlight the ability of altered variants of central carbon metabolism to arise in response to unique environments.
Figures

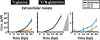
Comment in
-
Metabolism: Malaria parasite stands out.Nature. 2010 Aug 5;466(7307):702-3. doi: 10.1038/466702a. Nature. 2010. PMID: 20686561 No abstract available.
References
-
- Krebs HA, Johnson WA. The role of citric acid in intermediate metabolism in animal tissues. Enzymologia. 1937;4:148. - PubMed
-
- van Dooren GG, Stimmler LM, McFadden GI. Metabolic maps and functions of the Plasmodium mitochondrion. FEMS Microbiol Rev. 2006;30(4):596. - PubMed
-
- Sherman IW. In: Malaria, Parasite Biology, Pathogenesis and Protection. Sherman IW, editor. ASM Press; Washington, D.C.: 1998. p. 135.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

