Memory consolidation in the cerebellar cortex
- PMID: 20686596
- PMCID: PMC2912226
- DOI: 10.1371/journal.pone.0011737
Memory consolidation in the cerebellar cortex
Abstract
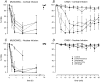
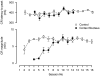
Several forms of learning, including classical conditioning of the eyeblink, depend upon the cerebellum. In examining mechanisms of eyeblink conditioning in rabbits, reversible inactivations of the control circuitry have begun to dissociate aspects of cerebellar cortical and nuclear function in memory consolidation. It was previously shown that post-training cerebellar cortical, but not nuclear, inactivations with the GABAA agonist muscimol prevented consolidation but these findings left open the question as to how final memory storage was partitioned across cortical and nuclear levels. Memory consolidation might be essentially cortical and directly disturbed by actions of the muscimol, or it might be nuclear, and sensitive to the raised excitability of the nuclear neurons following the loss of cortical inhibition. To resolve this question, we simultaneously inactivated cerebellar cortical lobule HVI and the anterior interpositus nucleus of rabbits during the post-training period, so protecting the nuclei from disinhibitory effects of cortical inactivation. Consolidation was impaired by these simultaneous inactivations. Because direct application of muscimol to the nuclei alone has no impact upon consolidation, we can conclude that post-training, consolidation processes and memory storage for eyeblink conditioning have critical cerebellar cortical components. The findings are consistent with a recent model that suggests the distribution of learning-related plasticity across cortical and nuclear levels is task-dependent. There can be transfer to nuclear or brainstem levels for control of high-frequency responses but learning with lower frequency response components, such as in eyeblink conditioning, remains mainly dependent upon cortical memory storage.
Conflict of interest statement
Figures





Similar articles
-
Cerebellar function in consolidation of a motor memory.Neuron. 2002 Jun 13;34(6):1011-20. doi: 10.1016/s0896-6273(02)00719-5. Neuron. 2002. PMID: 12086647
-
Temporal properties of cerebellar-dependent memory consolidation.J Neurosci. 2004 Mar 24;24(12):2934-41. doi: 10.1523/JNEUROSCI.5505-03.2004. J Neurosci. 2004. PMID: 15044532 Free PMC article.
-
Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI.J Neurosci. 2001 Aug 1;21(15):5715-22. doi: 10.1523/JNEUROSCI.21-15-05715.2001. J Neurosci. 2001. PMID: 11466443 Free PMC article.
-
Neuroscience and learning: lessons from studying the involvement of a region of cerebellar cortex in eyeblink classical conditioning.J Exp Anal Behav. 2005 Nov;84(3):631-52. doi: 10.1901/jeab.2005.96-04. J Exp Anal Behav. 2005. PMID: 16596983 Free PMC article. Review.
-
Role of the nuclei in eyeblink conditioning.Ann N Y Acad Sci. 2002 Dec;978:93-105. doi: 10.1111/j.1749-6632.2002.tb07558.x. Ann N Y Acad Sci. 2002. PMID: 12582044 Review.
Cited by
-
Plasticity leading to cerebellum-dependent learning: two different regions, two different types.Pflugers Arch. 2019 Jul;471(7):927-934. doi: 10.1007/s00424-019-02282-3. Epub 2019 May 19. Pflugers Arch. 2019. PMID: 31104128 Review.
-
Modeling memory consolidation during posttraining periods in cerebellovestibular learning.Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):3541-6. doi: 10.1073/pnas.1413798112. Epub 2015 Mar 3. Proc Natl Acad Sci U S A. 2015. PMID: 25737547 Free PMC article.
-
Golgi cell activity during eyeblink conditioning in decerebrate ferrets.Cerebellum. 2014 Feb;13(1):42-5. doi: 10.1007/s12311-013-0518-3. Cerebellum. 2014. PMID: 23982588
-
Changes in complex spike activity during classical conditioning.Front Neural Circuits. 2014 Aug 5;8:90. doi: 10.3389/fncir.2014.00090. eCollection 2014. Front Neural Circuits. 2014. PMID: 25140129 Free PMC article.
-
Storage of a naturally acquired conditioned response is impaired in patients with cerebellar degeneration.Brain. 2013 Jul;136(Pt 7):2063-76. doi: 10.1093/brain/awt107. Epub 2013 May 31. Brain. 2013. PMID: 23729474 Free PMC article.
References
-
- Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609. - PubMed
-
- Ito M. Cerebellar control of the vestibulo-ocular reflex–around the flocculus hypothesis. Annu Rev Neurosci. 1982;5:275–296. - PubMed
-
- Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972;40:81–84. - PubMed
-
- Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–299. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

