Histological and functional benefit following transplantation of motor neuron progenitors to the injured rat spinal cord
- PMID: 20686613
- PMCID: PMC2912300
- DOI: 10.1371/journal.pone.0011852
Histological and functional benefit following transplantation of motor neuron progenitors to the injured rat spinal cord
Abstract
Background: Motor neuron loss is characteristic of cervical spinal cord injury (SCI) and contributes to functional deficit.
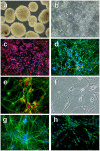
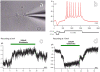
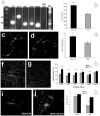
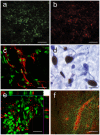
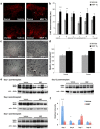
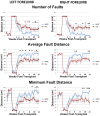
Methodology/principal findings: In order to investigate the amenability of the injured adult spinal cord to motor neuron differentiation, we transplanted spinal cord injured animals with a high purity population of human motor neuron progenitors (hMNP) derived from human embryonic stem cells (hESCs). In vitro, hMNPs displayed characteristic motor neuron-specific markers, a typical electrophysiological profile, functionally innervated human or rodent muscle, and secreted physiologically active growth factors that caused neurite branching and neuronal survival. hMNP transplantation into cervical SCI sites in adult rats resulted in suppression of intracellular signaling pathways associated with SCI pathogenesis, which correlated with greater endogenous neuronal survival and neurite branching. These neurotrophic effects were accompanied by significantly enhanced performance on all parameters of the balance beam task, as compared to controls. Interestingly, hMNP transplantation resulted in survival, differentiation, and site-specific integration of hMNPs distal to the SCI site within ventral horns, but hMNPs near the SCI site reverted to a neuronal progenitor state, suggesting an environmental deficiency for neuronal maturation associated with SCI.
Conclusions/significance: These findings underscore the barriers imposed on neuronal differentiation of transplanted cells by the gliogenic nature of the injured spinal cord, and the physiological relevance of transplant-derived neurotrophic support to functional recovery.
Conflict of interest statement
Figures
Similar articles
-
Repair of spinal cord injury by co-transplantation of embryonic stem cell-derived motor neuron and olfactory ensheathing cell.Iran Biomed J. 2009 Jul;13(3):125-35. Iran Biomed J. 2009. PMID: 19688018
-
Effects of glial transplantation on functional recovery following acute spinal cord injury.J Neurotrauma. 2005 May;22(5):575-89. doi: 10.1089/neu.2005.22.575. J Neurotrauma. 2005. PMID: 15892602
-
Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat.Acta Neurochir (Wien). 2005 Sep;147(9):985-92; discussion 992. doi: 10.1007/s00701-005-0538-y. Epub 2005 Jul 11. Acta Neurochir (Wien). 2005. PMID: 16010451
-
Human stem cell-derived neurons and neural circuitry therapeutics: Next frontier in spinal cord injury repair.Exp Biol Med (Maywood). 2022 Dec;247(23):2142-2151. doi: 10.1177/15353702221114099. Epub 2022 Aug 16. Exp Biol Med (Maywood). 2022. PMID: 35974701 Free PMC article. Review.
-
The role of embryonic motoneuron transplants to restore the lost motor function of the injured spinal cord.Ann Anat. 2011 Jul;193(4):362-70. doi: 10.1016/j.aanat.2011.04.001. Epub 2011 Apr 30. Ann Anat. 2011. PMID: 21600746 Review.
Cited by
-
Cell therapy and delivery strategies for spinal cord injury.Histol Histopathol. 2021 Sep;36(9):907-920. doi: 10.14670/HH-18-350. Epub 2021 Jun 10. Histol Histopathol. 2021. PMID: 34109994 Review.
-
Transplantation of gene-corrected motor neurons as a therapeutic strategy for spinal muscular atrophy.Mol Ther. 2013 Mar;21(3):502-3. doi: 10.1038/mt.2013.23. Mol Ther. 2013. PMID: 23449105 Free PMC article. No abstract available.
-
Long-Term Effects of Neural Precursor Cell Transplantation on Secondary Injury Processes and Functional Recovery after Severe Cervical Contusion-Compression Spinal Cord Injury.Int J Mol Sci. 2021 Dec 3;22(23):13106. doi: 10.3390/ijms222313106. Int J Mol Sci. 2021. PMID: 34884911 Free PMC article.
-
Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis.Cell Res. 2011 Nov;21(11):1551-63. doi: 10.1038/cr.2011.148. Epub 2011 Sep 6. Cell Res. 2011. PMID: 21894191 Free PMC article.
-
Derivation and Identification of Motor Neurons from Human Urine-Derived Induced Pluripotent Stem Cells.Stem Cells Int. 2018 Jan 24;2018:3628578. doi: 10.1155/2018/3628578. eCollection 2018. Stem Cells Int. 2018. PMID: 29531534 Free PMC article.
References
-
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. - PubMed
-
- Zhang YW, Denham J, Thies RS. Oligodendrocyte Progenitor Cells Derived from Human Embryonic Stem Cells Express Neurotrophic Factors. Stem Cells and Development. 2006;15:943–952. - PubMed

