Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells
- PMID: 20686615
- PMCID: PMC2912324
- DOI: 10.1371/journal.pone.0011853
Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells
Abstract
Background: Directed differentiation of human induced pluripotent stem cells (hiPSC) into functional, region-specific neural cells is a key step to realizing their therapeutic promise to treat various neural disorders, which awaits detailed elucidation.
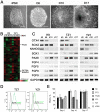
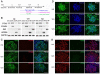
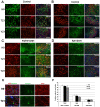
Methodology/principal findings: We analyzed neural differentiation from various hiPSC lines generated by others and ourselves. Although heterogeneity in efficiency of neuroepithelial (NE) cell differentiation was observed among different hiPSC lines, the NE differentiation process resembles that from human embryonic stem cells (hESC) in morphology, timing, transcriptional profile, and requirement for FGF signaling. NE cells differentiated from hiPSC, like those from hESC, can also form rostral phenotypes by default, and form the midbrain or spinal progenitors upon caudalization by morphogens. The rostrocaudal neural progenitors can further mature to develop forebrain glutamatergic projection neurons, midbrain dopaminergic neurons, and spinal motor neurons, respectively. Typical ion channels and action potentials were recorded in the hiPSC-derived neurons.
Conclusions/significance: Our results demonstrate that hiPSC, regardless of how they were derived, can differentiate into a spectrum of rostrocaudal neurons with functionality, which supports the considerable value of hiPSC for study and treatment of patient-specific neural disorders.
Conflict of interest statement
Figures


Similar articles
-
Optogenetics reveal delayed afferent synaptogenesis on grafted human-induced pluripotent stem cell-derived neural progenitors.Stem Cells. 2014 Dec;32(12):3088-98. doi: 10.1002/stem.1823. Stem Cells. 2014. PMID: 25183299
-
Differentiation and Characterization of Dopaminergic Neurons From Baboon Induced Pluripotent Stem Cells.Stem Cells Transl Med. 2016 Sep;5(9):1133-44. doi: 10.5966/sctm.2015-0073. Epub 2016 Jun 24. Stem Cells Transl Med. 2016. PMID: 27343168 Free PMC article.
-
Specification of murine ground state pluripotent stem cells to regional neuronal populations.Sci Rep. 2017 Nov 22;7(1):16001. doi: 10.1038/s41598-017-16248-x. Sci Rep. 2017. PMID: 29167563 Free PMC article.
-
Induction of early neural precursors and derivation of tripotent neural stem cells from human pluripotent stem cells under xeno-free conditions.J Comp Neurol. 2014 Aug 15;522(12):2767-83. doi: 10.1002/cne.23604. Epub 2014 Apr 25. J Comp Neurol. 2014. PMID: 24715528
-
Human stem cell models of dementia.Hum Mol Genet. 2014 Sep 15;23(R1):R35-9. doi: 10.1093/hmg/ddu302. Epub 2014 Jun 16. Hum Mol Genet. 2014. PMID: 24939911 Free PMC article. Review.
Cited by
-
Astroglial cells regulate the developmental timeline of human neurons differentiated from induced pluripotent stem cells.Stem Cell Res. 2013 Sep;11(2):743-57. doi: 10.1016/j.scr.2013.05.002. Epub 2013 May 16. Stem Cell Res. 2013. PMID: 23759711 Free PMC article.
-
Aβ Assemblies Promote Amyloidogenic Processing of APP and Intracellular Accumulation of Aβ42 Through Go/Gβγ Signaling.Front Cell Dev Biol. 2022 Apr 4;10:852738. doi: 10.3389/fcell.2022.852738. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35445022 Free PMC article.
-
Functional coupling of TRPM2 and extrasynaptic NMDARs exacerbates excitotoxicity in ischemic brain injury.Neuron. 2022 Jun 15;110(12):1944-1958.e8. doi: 10.1016/j.neuron.2022.03.021. Epub 2022 Apr 13. Neuron. 2022. PMID: 35421327 Free PMC article.
-
Decoding the synaptic dysfunction of bioactive human AD brain soluble Aβ to inspire novel therapeutic avenues for Alzheimer's disease.Acta Neuropathol Commun. 2018 Nov 8;6(1):121. doi: 10.1186/s40478-018-0626-x. Acta Neuropathol Commun. 2018. PMID: 30409172 Free PMC article.
-
Hedgehog Promotes Production of Inhibitory Interneurons in Vivo and in Vitro from Pluripotent Stem Cells.J Dev Biol. 2016 Aug 26;4(3):26. doi: 10.3390/jdb4030026. J Dev Biol. 2016. PMID: 29615590 Free PMC article. Review.
References
-
- Martins-Taylor K, Xu RH. Determinants of pluripotency: From avian, rodents, to primates. J Cell Biochem. 2009;109:16–25. - PubMed
-
- Zhang SC. Embryonic stem cells for neural replacement therapy: prospects and challenges. J Hematother Stem Cell Res. 2003;12:625–634. - PubMed
-
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. - PubMed
-
- Li XJ, Zhang SC. In vitro differentiation of neural precursors from human embryonic stem cells. Methods Mol Biol. 2006;331:169–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

