Plasmodium falciparum adhesion on human brain microvascular endothelial cells involves transmigration-like cup formation and induces opening of intercellular junctions
- PMID: 20686652
- PMCID: PMC2912387
- DOI: 10.1371/journal.ppat.1001021
Plasmodium falciparum adhesion on human brain microvascular endothelial cells involves transmigration-like cup formation and induces opening of intercellular junctions
Abstract
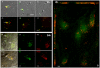
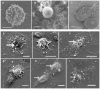
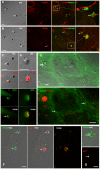
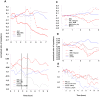
Cerebral malaria, a major cause of death during malaria infection, is characterised by the sequestration of infected red blood cells (IRBC) in brain microvessels. Most of the molecules implicated in the adhesion of IRBC on endothelial cells (EC) are already described; however, the structure of the IRBC/EC junction and the impact of this adhesion on the EC are poorly understood. We analysed this interaction using human brain microvascular EC monolayers co-cultured with IRBC. Our study demonstrates the transfer of material from the IRBC to the brain EC plasma membrane in a trogocytosis-like process, followed by a TNF-enhanced IRBC engulfing process. Upon IRBC/EC binding, parasite antigens are transferred to early endosomes in the EC, in a cytoskeleton-dependent process. This is associated with the opening of the intercellular junctions. The transfer of IRBC antigens can thus transform EC into a target for the immune response and contribute to the profound EC alterations, including peri-vascular oedema, associated with cerebral malaria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Binding Heterogeneity of Plasmodium falciparum to Engineered 3D Brain Microvessels Is Mediated by EPCR and ICAM-1.mBio. 2019 May 28;10(3):e00420-19. doi: 10.1128/mBio.00420-19. mBio. 2019. PMID: 31138740 Free PMC article.
-
Selection of Plasmodium falciparum parasites for cytoadhesion to human brain endothelial cells.J Vis Exp. 2012 Jan 3;(59):e3122. doi: 10.3791/3122. J Vis Exp. 2012. PMID: 22230803 Free PMC article.
-
Inhibition of Infected Red Blood Cell Binding to the Vascular Endothelium.Methods Mol Biol. 2015;1325:215-29. doi: 10.1007/978-1-4939-2815-6_18. Methods Mol Biol. 2015. PMID: 26450392
-
The role of ICAM-1 in Plasmodium falciparum cytoadherence.Eur J Cell Biol. 2005 Jan;84(1):15-27. doi: 10.1016/j.ejcb.2004.09.002. Eur J Cell Biol. 2005. PMID: 15724813 Review.
-
Variant antigens of Plasmodium falciparum encoded by the var multigenic family are multifunctional macromolecules.Res Microbiol. 2001 Mar;152(2):141-7. doi: 10.1016/s0923-2508(01)01184-6. Res Microbiol. 2001. PMID: 11316367 Review.
Cited by
-
The Complement System Is Essential for the Phagocytosis of Mesenchymal Stromal Cells by Monocytes.Front Immunol. 2019 Sep 20;10:2249. doi: 10.3389/fimmu.2019.02249. eCollection 2019. Front Immunol. 2019. PMID: 31616424 Free PMC article.
-
Malaria: modification of the red blood cell and consequences in the human host.Br J Haematol. 2011 Sep;154(6):670-9. doi: 10.1111/j.1365-2141.2011.08755.x. Epub 2011 May 28. Br J Haematol. 2011. PMID: 21623767 Free PMC article. Review.
-
Inhibition of hypoxia-associated response and kynurenine production in response to hyperbaric oxygen as mechanisms involved in protection against experimental cerebral malaria.FASEB J. 2018 Aug;32(8):4470-4481. doi: 10.1096/fj.201700844R. Epub 2018 Mar 20. FASEB J. 2018. PMID: 29558201 Free PMC article.
-
Unravelling mysteries at the perivascular space: a new rationale for cerebral malaria pathogenesis.Trends Parasitol. 2024 Jan;40(1):28-44. doi: 10.1016/j.pt.2023.11.005. Epub 2023 Dec 8. Trends Parasitol. 2024. PMID: 38065791 Free PMC article. Review.
-
Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria.EMBO Mol Med. 2013 Jul;5(7):984-99. doi: 10.1002/emmm.201202273. Epub 2013 May 16. EMBO Mol Med. 2013. PMID: 23681698 Free PMC article.
References
-
- WHO. 2008. World malaria report 2008. www.who.org.
-
- Newton CR, Warn PA, Winstanley PA, Peshu N, Snow RW, et al. Severe anaemia in children living in a malaria endemic area of Kenya. Trop Med Int Health. 1997;2:165–178. - PubMed
-
- Deininger MH, Kremsner PG, Meyermann R, Schluesener HJ. Focal accumulation of cyclooxygenase-1 (COX-1) and COX-2 expressing cells in cerebral malaria. J Neuroimmunol. 2000;106:198–205. - PubMed
-
- Saavedra-Lozano J, Booth TN, Weprin BE, Ramilo O. Isolated cerebellar edema and obstructive hydrocephalus in a child with cerebral malaria. Pediatr Infect Dis. 2001;20:908–911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

