Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature
- PMID: 20686684
- PMCID: PMC2912336
- DOI: 10.1371/journal.pone.0011863
Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature
Abstract
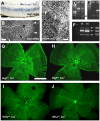
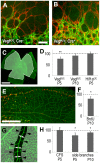
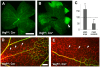
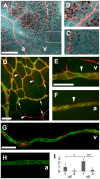
Vascular endothelial growth factor (VEGF) plays a critical role in normal development as well as retinal vasculature disease. During retinal vascularization, VEGF is most strongly expressed by not yet vascularized retinal astrocytes, but also by retinal astrocytes within the developing vascular plexus, suggesting a role for retinal astrocyte-derived VEGF in angiogenesis and vessel network maturation. To test the role of astrocyte-derived VEGF, we used Cre-lox technology in mice to delete VEGF in retinal astrocytes during development. Surprisingly, this only had a minor impact on retinal vasculature development, with only small decreases in plexus spreading, endothelial cell proliferation and survival observed. In contrast, astrocyte VEGF deletion had more pronounced effects on hyperoxia-induced vaso-obliteration and led to the regression of smooth muscle cell-coated radial arteries and veins, which are usually resistant to the vessel-collapsing effects of hyperoxia. These results suggest that VEGF production from retinal astrocytes is relatively dispensable during development, but performs vessel stabilizing functions in the retinal vasculature and might be relevant for retinopathy of prematurity in humans.
Conflict of interest statement
Figures
Similar articles
-
Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina.Glia. 2010 Aug;58(10):1177-85. doi: 10.1002/glia.20997. Glia. 2010. PMID: 20544853 Free PMC article.
-
Bit1-a novel regulator of astrocyte function during retinal development: proliferation, migration, and paracrine effects on vascular endothelial cell.Hum Cell. 2019 Oct;32(4):418-427. doi: 10.1007/s13577-019-00272-2. Epub 2019 Jul 31. Hum Cell. 2019. PMID: 31368047
-
Hyperoxia causes reduced density of retinal astrocytes in the central avascular zone in the mouse model of oxygen-induced retinopathy.Mol Cell Neurosci. 2013 Sep;56:225-33. doi: 10.1016/j.mcn.2013.06.001. Epub 2013 Jun 10. Mol Cell Neurosci. 2013. PMID: 23756201
-
Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review.
-
Seeing stars: Development and function of retinal astrocytes.Dev Biol. 2021 Oct;478:144-154. doi: 10.1016/j.ydbio.2021.07.007. Epub 2021 Jul 11. Dev Biol. 2021. PMID: 34260962 Free PMC article. Review.
Cited by
-
Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling.PLoS Biol. 2013;11(1):e1001469. doi: 10.1371/journal.pbio.1001469. Epub 2013 Jan 22. PLoS Biol. 2013. PMID: 23349620 Free PMC article.
-
Neural Regulation of CNS Angiogenesis During Development.Front Biol (Beijing). 2015 Feb;10(1):61-73. doi: 10.1007/s11515-014-1331-y. Front Biol (Beijing). 2015. PMID: 25691893 Free PMC article.
-
Proteomic study of aqueous humour in diabetic patients with cataracts by TMT combined with HPLC-MS/MS.BMC Ophthalmol. 2023 Oct 26;23(1):435. doi: 10.1186/s12886-023-03162-2. BMC Ophthalmol. 2023. PMID: 37884923 Free PMC article.
-
Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis.Development. 2011 Oct;138(20):4451-63. doi: 10.1242/dev.071381. Epub 2011 Aug 31. Development. 2011. PMID: 21880786 Free PMC article.
-
Cyp1b1-deficient retinal astrocytes are more proliferative and migratory and are protected from oxidative stress and inflammation.Am J Physiol Cell Physiol. 2019 Jun 1;316(6):C767-C781. doi: 10.1152/ajpcell.00021.2019. Epub 2019 Mar 20. Am J Physiol Cell Physiol. 2019. PMID: 30892936 Free PMC article.
References
-
- Alon T, Hemo I, Itin A, Pe'er J, Stone J, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. - PubMed
-
- Fruttiger M, Karlsson L, Hall AC, Abramsson A, Calver AR, et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. - PubMed
-
- Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci. 2002;43:522–527. - PubMed
-
- Schnitzer J. Astrocytes in the guinea pig, horse, and monkey retina: their occurrence coincides with the presence of blood vessels. Glia. 1988;1:74–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

