A double-blind, randomized, multicenter, Italian study of frovatriptan versus rizatriptan for the acute treatment of migraine
- PMID: 20686810
- PMCID: PMC3075392
- DOI: 10.1007/s10194-010-0243-y
A double-blind, randomized, multicenter, Italian study of frovatriptan versus rizatriptan for the acute treatment of migraine
Abstract
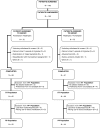
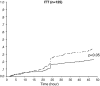
The objective of this study was to assess patient satisfaction with acute treatment of migraine with frovatriptan or rizatriptan by preference questionnaire. 148 subjects with a history of migraine with or without aura (IHS 2004 criteria), with at least one migraine attack per month in the preceding 6 months, were enrolled and randomized to frovatriptan 2.5 mg or rizatriptan 10 mg treating 1-3 attacks. The study had a multicenter, randomized, double-blind, cross-over design, with treatment periods lasting <3 months. At the end of the study, patients assigned preference to one of the treatments using a questionnaire with a score from 0 to 5 (primary endpoint). Secondary endpoints were pain-free and pain relief episodes at 2 h, and recurrent and sustained pain-free episodes within 48 h. 104 of the 125 patients (83%, intention-to-treat population) expressed a preference for a triptan. The average preference score was not significantly different between frovatriptan (2.9±1.3) and rizatriptan (3.2±1.1). The rates of pain-free (33% frovatriptan vs. 39% rizatriptan) and pain relief (55 vs. 62%) episodes at 2 h were not significantly different between the two treatments. The rate of recurrent episodes was significantly (p<0.001) lower under frovatriptan (21 vs. 43% rizatriptan). No significant differences were observed in sustained pain-free episodes (26% frovatriptan vs. 22% rizatriptan). The number of patients with adverse events was not significantly different between rizatriptan (34) and frovatriptan (25, p=NS). The results suggest that frovatriptan has a similar efficacy to rizatriptan, but a more prolonged duration of action.
© Springer-Verlag 2010
Figures
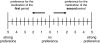
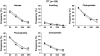
Comment in
-
When to use frovatriptan in migraine?J Headache Pain. 2011 Jun;12(3):393-4; author reply 395-6. doi: 10.1007/s10194-011-0341-5. Epub 2011 Apr 22. J Headache Pain. 2011. PMID: 21512776 Free PMC article. No abstract available.
-
Suggested randomised, controlled trial with frovatriptan.J Headache Pain. 2011 Dec;12(6):665-6; author reply 663-4. doi: 10.1007/s10194-011-0381-x. Epub 2011 Sep 14. J Headache Pain. 2011. PMID: 21915651 Free PMC article. No abstract available.
Similar articles
-
Efficacy of frovatriptan and other triptans in the treatment of acute migraine of hypertensive and normotensive subjects: a review of randomized studies.Neurol Sci. 2013 May;34 Suppl 1:S87-91. doi: 10.1007/s10072-013-1367-z. Neurol Sci. 2013. PMID: 23695053 Review.
-
Efficacy of frovatriptan in the acute treatment of menstrually related migraine: analysis of a double-blind, randomized, cross-over, multicenter, Italian, comparative study versus rizatriptan.J Headache Pain. 2011 Dec;12(6):609-15. doi: 10.1007/s10194-011-0366-9. Epub 2011 Aug 13. J Headache Pain. 2011. PMID: 21842274 Free PMC article. Clinical Trial.
-
Efficacy and pharmacokinetic activity of frovatriptan compared to rizatriptan in patients with moderate-to-severe migraine.Drug Des Devel Ther. 2014 Jul 21;8:983-92. doi: 10.2147/DDDT.S61295. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25092964 Free PMC article. Clinical Trial.
-
A double-blind, randomized, multicenter, Italian study of frovatriptan versus almotriptan for the acute treatment of migraine.J Headache Pain. 2011 Jun;12(3):361-8. doi: 10.1007/s10194-011-0325-5. Epub 2011 Mar 25. J Headache Pain. 2011. PMID: 21437714 Free PMC article. Clinical Trial.
-
Gender and triptan efficacy: a pooled analysis of three double-blind, randomized, crossover, multicenter, Italian studies comparing frovatriptan vs. other triptans.Neurol Sci. 2014 May;35 Suppl 1(Suppl 1):99-105. doi: 10.1007/s10072-014-1750-4. Neurol Sci. 2014. PMID: 24867845 Free PMC article. Review.
Cited by
-
Efficacy of frovatriptan and other triptans in the treatment of acute migraine of hypertensive and normotensive subjects: a review of randomized studies.Neurol Sci. 2013 May;34 Suppl 1:S87-91. doi: 10.1007/s10072-013-1367-z. Neurol Sci. 2013. PMID: 23695053 Review.
-
Frovatriptan and rizatriptan economic EVAluation: the FREEVA study.J Headache Pain. 2013 Dec 11;14(1):96. doi: 10.1186/1129-2377-14-96. J Headache Pain. 2013. PMID: 24330707 Free PMC article. Clinical Trial.
-
Efficacy of frovatriptan and other triptans in the treatment of acute migraine of normal weight and obese subjects: a review of randomized studies.Neurol Sci. 2014 May;35 Suppl 1:115-9. doi: 10.1007/s10072-014-1752-2. Neurol Sci. 2014. PMID: 24867847 Review.
-
Predicting the response to a triptan in migraine using deep attack phenotyping: A feasibility study.Cephalalgia. 2021 Feb;41(2):197-202. doi: 10.1177/0333102420959786. Epub 2020 Sep 21. Cephalalgia. 2021. PMID: 32955929 Free PMC article.
-
Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: Fourth edition.Cephalalgia. 2019 May;39(6):687-710. doi: 10.1177/0333102419828967. Epub 2019 Feb 26. Cephalalgia. 2019. PMID: 30806518 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

