Proteases in cutaneous malignant melanoma: relevance as biomarker and therapeutic target
- PMID: 20686912
- PMCID: PMC11115755
- DOI: 10.1007/s00018-010-0469-5
Proteases in cutaneous malignant melanoma: relevance as biomarker and therapeutic target
Abstract
Cutaneous malignant melanoma is the most aggressive skin cancer. It is also the most rapidly spreading cancer in terms of worldwide incidence. Although it is detected by simple inspection and can be relatively easily removed or treated, differential diagnosis to other melanocytic lesions, lack of prognostic markers, and no efficient treatment of advanced melanoma pose problems. Detection and targeting of proteases may represent a useful tool since they play a role in tumor cell metabolism, invasion, angiogenesis and metastasis. This review gives an overview of the role of proteases in development and progression of cutaneous malignant melanoma. In addition, regulation, activation, and interaction of proteases and their inhibitors are explained for tumors in general. The potential use of proteases as differential markers for melanoma mimicking melanocytic lesions, as biomarkers in tissues, and as prognostic serum markers is discussed. Current and future possibilities to target tumor proteases in therapy are presented.
Figures

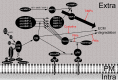
 ). Activation of pro-CB is achieved by cathepsins D and G and by the tissue plasminogen activator system (see Fig. 2 for more detail). Extra Extracellular space, PM plasma membrane, Intra intracellular space
). Activation of pro-CB is achieved by cathepsins D and G and by the tissue plasminogen activator system (see Fig. 2 for more detail). Extra Extracellular space, PM plasma membrane, Intra intracellular spaceSimilar articles
-
Proteases in cutaneous melanoma.Ann Med. 1998 Oct;30(5):431-42. doi: 10.3109/07853899809002484. Ann Med. 1998. PMID: 9814829 Review.
-
Pathology of malignant melanoma, including new markers and techniques in diagnosis and prognosis.Curr Opin Oncol. 1996 Mar;8(2):143-51. doi: 10.1097/00001622-199603000-00012. Curr Opin Oncol. 1996. PMID: 8727307 Review.
-
Melanocytic schwannoma of the cutaneous and subcutaneous tissues: three cases and a review of the literature.Melanoma Res. 2008 Dec;18(6):438-42. doi: 10.1097/CMR.0b013e32831270d7. Melanoma Res. 2008. PMID: 19011514 Review.
-
Serum matrix metalloproteinase-2 as a prognostic marker in advanced cutaneous melanoma.Acta Oncol. 2000;39(7):877-9. doi: 10.1080/028418600750063659. Acta Oncol. 2000. PMID: 11145448
-
Inducible nitric oxide synthase expression in benign and malignant cutaneous melanocytic lesions.J Pathol. 2001 Jun;194(2):194-200. doi: 10.1002/1096-9896(200106)194:2<194::AID-PATH851>3.0.CO;2-S. J Pathol. 2001. PMID: 11400148
Cited by
-
Aberrant expression of kallikrein-related peptidase 7 is correlated with human melanoma aggressiveness by stimulating cell migration and invasion.Mol Oncol. 2017 Oct;11(10):1330-1347. doi: 10.1002/1878-0261.12103. Epub 2017 Aug 11. Mol Oncol. 2017. PMID: 28636767 Free PMC article.
-
Wogonin suppresses melanoma cell B16-F10 invasion and migration by inhibiting Ras-medicated pathways.PLoS One. 2014 Sep 9;9(9):e106458. doi: 10.1371/journal.pone.0106458. eCollection 2014. PLoS One. 2014. PMID: 25203554 Free PMC article.
-
Cysteine cathepsins: their role in tumor progression and recent trends in the development of imaging probes.Front Chem. 2015 Jun 23;3:37. doi: 10.3389/fchem.2015.00037. eCollection 2015. Front Chem. 2015. PMID: 26157794 Free PMC article. Review.
-
Matrix metalloproteinase-3 gene promoter polymorphisms: A potential risk factor for pelvic organ prolapse.Biomed Rep. 2016 Sep;5(3):337-343. doi: 10.3892/br.2016.736. Epub 2016 Aug 3. Biomed Rep. 2016. PMID: 27588175 Free PMC article.
-
Investigation of the role of MMP3 -1171insA polymorphism in cutaneous malignant melanoma - a preliminary study.Biotechnol Biotechnol Equip. 2014 Sep 3;28(5):904-910. doi: 10.1080/13102818.2014.947694. Epub 2014 Nov 13. Biotechnol Biotechnol Equip. 2014. PMID: 26019576 Free PMC article.
References
-
- Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(Suppl 61):1–6. - PubMed
-
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–185. - PubMed
-
- Buzaid AC, Anderson CM. The changing prognosis of melanoma. Curr Oncol Rep. 2000;2:322–328. - PubMed
-
- MacKie RM. Malignant melanoma: clinical variants and prognostic indicators. Clin Exp Dermatol. 2000;25:471–475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

