Vaccines for preventing cholera: killed whole cell or other subunit vaccines (injected)
- PMID: 20687062
- PMCID: PMC6532721
- DOI: 10.1002/14651858.CD000974.pub2
Vaccines for preventing cholera: killed whole cell or other subunit vaccines (injected)
Abstract
Background: Injected cholera vaccines are rarely used today, although they may have some benefit. It is valuable to summarize the evidence for effectiveness of injected cholera vaccines for comparison with newer oral vaccines (subject of a separate Cochrane Review).
Objectives: To evaluate killed whole cell (KWC) cholera vaccines and other inactive subunit vaccines (administered by injection) for preventing cholera and death, and to evaluate the adverse effects.
Search strategy: In September 2008, we searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (The Cochrane Library 2008, Issue 3), EMBASE, and LILACS. We also searched reference lists and handsearched the journal Vaccine up to 1997.
Selection criteria: Randomized and quasi-randomized controlled trials comparing injected cholera vaccines (KWC or other inactive subunit) with placebo, control vaccines, or no intervention in adults and children irrespective of immune status or special risk category.
Data collection and analysis: Two authors extracted data and assessed trial methodological quality independently. Dichotomous data were reported using the risk ratio (RR) with 95% confidence intervals (CI). Vaccine efficacies were also calculated (% vaccine efficacy = (1-RR) x 100%).
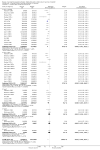
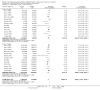
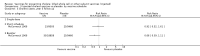
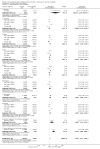
Main results: Sixteen trials, involving over one million adults, children and infants, fulfilled the inclusion criteria. Twenty-four comparisons reported on vaccine efficacy (cholera cases and/or deaths) and 11 comparisons considered adverse effects (nine reported on both). Compared to placebo, vaccinees had a reduced risk of death from cholera (RR 0.49, 95% CI 0.25 to 0.93; 837,442 participants) and a reduced risk of contracting cholera at 12 months (RR 0.52, 95% CI 0.42 to 0.65, random-effects model; 1,512,573 participants). This translates to an efficacy of 48%, 95% confidence interval 35% to 58%. Significant protection lasted for two years, even after only a single dose, and for three years with an annual booster. Children over five years and adults were protected for up to three years, while children under five years were protected for up to a year. Injected cholera vaccines were associated with more systemic and local adverse effects compared to placebo, but these were not severe or life-threatening.
Authors' conclusions: Injected cholera vaccines appear to be safe and relatively more effective than usually realized. Protection against cholera persists for up to two years following a single dose of vaccine, and for three years with an annual booster. However, they have been superseded by oral vaccines.
Conflict of interest statement
None known.
Figures












Update of
-
Vaccines for preventing cholera.Cochrane Database Syst Rev. 2000;(4):CD000974. doi: 10.1002/14651858.CD000974. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2010 Aug 04;(8):CD000974. doi: 10.1002/14651858.CD000974.pub2. PMID: 11034693 Updated.
References
References to studies included in this review
Azurin 1965i {published data only}
-
- Azurin JC. A controlled field trial on the effectiveness of cholera and cholera el Tor vaccines in the Phillipines. Proceedings of the Cholera Research Symposium. 1965:369‐73.
Azurin 1965ii {published data only}
-
- See Azurin 1965i.
Azurin 1965iii {published data only}
-
- See Azurin 1965i.
Benenson 1968a {published data only}
Benenson 1968b‐i {published data only}
-
- Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural east Pakistan 2. A comparison of antibody titres in the immunized and control populations of a cholera‐vaccine field trial area and the relation of antibody titre to cholera case rate. Bulletin of the World Health Organization 1968;38(3):335‐46. - PMC - PubMed
Benenson 1968b‐ii {published data only}
-
- See Benenson 1968‐i.
Burgasov 1976 {published data only}
Curlin 1975 {unpublished data only}
-
- Curlin G, Levine M, Aziz KMA, Mizanur Rahman ASM, Verwey WF. Field trial of cholera toxoid. Proceedings of the eleventh joint conference on cholera. New Orleans: US‐Japan Cooperative Medical Science Program, 1975 Nov:314‐29.
-
- Curlin GT, Levine RJ, Ahmed A, Aziz KMA, Mizanur Rahman ASM, Verwey WF. Immunological aspects of a cholera toxoid field trial in Bangladesh. Dacca: Cholera Research Laboratory, 1978 March. Scientific Report No. 8.
das Gupta 1965a {published data only}
das Gupta 1965b‐i {published data only}
das Gupta 1965b‐ii {published data only}
-
- See das Gupta A 1965b‐i.
McCormack 1969 {published data only}
-
- McCormack WM, Rahman ASMM, Chowdhury AKMA, Mosley WH, Phillips RA. Report of the 1966‐67 cholera vaccine trial in rural East Pakistan 3. The lack of effect of prior vaccination or circulating vibriocidal antibody on the severity of clinical cholera. Bulletin of the World Health Organization 1969;40(2):199‐204. - PMC - PubMed
-
- Mosley WH, Aziz KMA, Rahman ASMM, Chowdhury AKMA, Ahmed A, Fahimuddin M. Report of the 1966‐67 cholera vaccine trial in rural East Pakistan. 4. Five years of observation with a practical assessment of the role of a cholera vaccine in cholera control programmes. Bulletin of the World Health Organization 1972;47(2):229‐38. - PMC - PubMed
-
- Mosley WH, McCormack WM, Ahmed A, Chowdhury AKMA, Barui RK. Report of the 1966‐67 cholera vaccine field trial in rural East Pakistan. 2. Results of the serological surveys in the study population‐‐the relationship of case rate to antibody titre and an estimate of the inapparent infection rate with Vibrio cholerae. Bulletin of the World Health Organization 1969;40(2):187‐97. - PMC - PubMed
Mosley 1970‐i {published data only}
-
- Mosley WH, Woodward WE, Aziz KM, Rahman AS, Chowdhury AK, Ahmed A, et al. The 1968‐1969 cholera‐vaccine field trial in rural East Pakistan. Effectiveness of monovalent Ogawa and Inaba vaccines and a purified Inaba antigen, with comparative results of serological and animal protection tests. Journal of Infectious Diseases 1970;121 Suppl:1‐9. - PubMed
Mosley 1970‐ii {published data only}
-
- See Mosley 1970‐i.
Mosley 1970‐iii {published data only}
-
- See Mosley 1970‐i.
Oseasohn 1965 {published data only}
-
- Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural east Pakistan. 2. A comparison of antibody titres in the immunized and control population of a cholera‐vaccine field‐trial area and the relation of antibody titre to cholera case rate. Bulletin of the World Health Organization 1968;38(3):335‐46. - PMC - PubMed
-
- Oseasohn RO, Benenson AS, Fahimuddin M. Cholera vaccine field trial in rural East Pakistan (first year of observation). Proceedings of the Cholera Research Symposium; 1965; Honolulu. 1965:362‐6.
Pal 1980 {published data only}
PCC 1968 {published data only}
PCC 1973a‐i {published data only}
PCC 1973a‐ii {published data only}
-
- See PCC 1973b‐i.
PCC 1973a‐iii {published data only}
-
- See PCC 1973b‐i.
PCC 1973a‐iv {published data only}
-
- See PCC 1973b‐i.
PCC 1973b {published data only}
Saroso 1978i {published data only}
Saroso 1978ii {published data only}
-
- See Saroso 1978i.
Taneja 1965 {published data only}
-
- Taneja BL. Controlled field trials of cholera vaccines in Calcutta. Proceedings of the Cholera Research Symposium. 1965:373‐379.
References to studies excluded from this review
Beran 1964 {published data only}
-
- Beran GW, Elvina O, Florendo FN Jr. Studies on the role of active immunization in the prevention of cholera El Tor. Journal of the Philippine Medical Association 1964;40(2):122‐35. - PubMed
Beran 1965 {published data only}
-
- Beran GW, Elviña O, Florendo FN Jr. Human volunteer studies using el tor cholera vaccines. I. Post‐vaccinal reactions. Journal of the Philippine Medical Association 1965;41(3):183‐90. - PubMed
Black 1979 {published data only}
-
- Black RE, Yunus MD, Eusof A, Ahmed A, Khan MR, Sack DA. Report on reactogenicity and immunogenicity of Wellcome cholera toxoids in Bangladeshi volunteers. Dacca, Bangladesh: International Centre for Diarrhoeal Disease Research, 1979 July. Scientific Report No. 29.
Cabrera 2005 {published data only}
-
- Cabrera O, Martínez ME, Cuello M, Soto CR, Valmaseda T, Cedré B, et al. Preparation and evaluation of vibrio cholerae O1 EL Tor Ogawa lipopolysaccharide‐tetanus toxoid conjugates. Vaccine 2006;2006(24 Suppl 2):74‐5. - PubMed
Chandra Sekar 1947 {published data only}
-
- Chandra Sekar C. Statistical assessment if the efficacy of anticholera inoculation from the data of 63 cheris in South Arcot district. Indian Journal of Medical Research 1947;35(3):153‐76.
Clasener 1968 {published data only}
-
- Clasener HA, Beunders BJ. Immunization of man with typhoid and cholera vaccine. Agglutinating antibodies after intracutaneous and subcutaneous injection. Acta Leidensia 1968;36:78‐85. - PubMed
Ganguly 1975 {published data only}
Gateff 1975 {published data only}
-
- Gateff C, Dodin A, Wiart J. A comparison of the serological effects of classical cholera vaccine and of purified fraction vaccine, with or without simultaneous yellow fever vaccine (author's transl). Annales de Microbiologie 1975;126(2):231‐46. - PubMed
Gupta 1998 {published data only}
McBean 1972 {published data only}
-
- McBean AM, Agle AN, Compaore P, Foster SO, McCormack WM. Comparison of intradermal and subcutaneous routes of cholera vaccine administration. Lancet 1972;1(7749):527‐9. - PubMed
Mosley 1968 {published data only}
-
- Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera‐vaccine field‐trial area and the relation of antibody titre to the pattern of endemic cholera. Bulletin of the World Health Organization 1968;38(3):327‐34. - PMC - PubMed
Nimbkar 1975 {published data only}
-
- Nimbkar YS, Karbhari RS, Cherian S, Chanderkar NG, Bhamaria RP, Ranadive PS, et al. Antibody response to two cholera vaccines in volunteers. Progress in Drug Research 1975;19:544‐62. - PubMed
Peltola 1977 {published data only}
-
- Peltola H, Ruutu P, Palomäki H, Kaukinen K, Aho K. Intracutaneous vaccination technic with less side effects against typhoid and cholera. Duodecim 1977;93(7):431‐8. - PubMed
Russell 1927 {published data only}
-
- Russell AJH. Bezredka's cholera bilivaccin versus anti‐cholera vaccine: a comparative field test. Transactions of the seventh congress of the Far East Association of Tropical Medicine 1927;1:523‐34.
Sommer 1973a {published data only}
-
- Sommer A, Khan M, Mosley WH. Efficacy of vaccination of family contacts of cholera cases. Lancet 1973;1(7814):1230‐2. - PubMed
Additional references
Abba (in progress)
Bhadra 1994
-
- Bhadra RK, Dasgupta U, Das J. Cholera vaccine: developmental strategies and problems. Indian Journal of Biochemistry and Biophysics 1994;31(6):441‐8. - PubMed
Calain 2004
-
- Calain P, Chaine JP, Johnson E, Hawley ML, O'Leary MJ, Oshitani H, et al. Can oral cholera vaccination play a role in controlling a cholera outbreak?. Vaccine 2004;22(19):2444‐51. - PubMed
Clemens 1994
-
- Clemens J, Spriggs D, Sack D. Public health considerations for the use of cholera vaccines in cholera control programes. In: Wachsmuth K, Blake PA, Olvik O editor(s). Vibrio cholerae and cholera: molecular to global perspectives. Washington: American Society for Microbiology, 1994:25‐40.
Clemens 1996
-
- Clemens J, Brenner R, Rao M, Tafari N, Lowe C. Evaluating new vaccines for developing countries. Efficacy or effectiveness?. JAMA 1996;275(5):390‐7. - PubMed
Cvjetanović 1978a
Cvjetanović 1978b
Davis 1995
-
- Davis R, Spencer CM. Live oral cholera vaccine: A preliminary review of its pharmacology and clinical potential in providing protective immunity against cholera. Clinical Immunotherapeutics 1995;4(3):235‐47.
Feeley 1978
-
- Feeley JC, Gangarosa EJ. Field trials of Cholera vaccine [Cholera and related diarrheas]. 43rd Nobel symposium; 1978; Stockholm, Sweden. Basel: Karger, 1980:204‐10.
Graves 2001
-
- Graves PM, Deeks JJ, Demicheli V, Pratt M, Jefferson T. Vaccines for preventing cholera. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000974] - DOI
Jefferson 1996
-
- Jefferson T, Jefferson V. The quest for trials on the efficacy of human vaccines. Results of the handsearch of Vaccine. Vaccine 1996;14(6):461‐4. - PubMed
Jefferson 1998
-
- Jefferson T. Vaccine trial data systematically assembled, pooled and disseminated by the Cochrane Collaboration. Vaccine 1998;16(16):1487‐95. - PubMed
Joo 1974
-
- Joo I. Cholera vaccines. In: Barua D, Burrows W editor(s). Cholera. Philadelphia: Saunders, 1974:333‐5.
Lefebvre 2008
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org (accessed 1 September 2008).
Sommer 1973b
-
- Sommer A, Mosley WH. Ineffectiveness of cholera vaccination as an epidemic control measure. Lancet 1973;814:1232‐5. - PubMed
Sánchez 1997
-
- Sánchez JL, Taylor DN. Cholera. Lancet 1997;349(9068):1825‐30. - PubMed
WHO 2006a
-
- WHO. Cholera, 2005. Weekly Epidemiological Record 2006;81:297‐308.
WHO 2006b
-
- World Health Organization, Global Task Force on Cholera Control. Oral cholera vaccine use in complex emergencies: what next? Report of a WHO meeting, 14‐16 December 2005, Cairo, Egypt [WHO/CDS/NTD/IDM/2006.2]. Geneva: World Health Organization, 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

