Interventions for treating oral mucositis for patients with cancer receiving treatment
- PMID: 20687070
- PMCID: PMC6669240
- DOI: 10.1002/14651858.CD001973.pub4
Interventions for treating oral mucositis for patients with cancer receiving treatment
Abstract
Background: Treatment of cancer is increasingly effective but associated with short and long term side effects. Oral side effects, including oral mucositis (mouth ulceration), remain a major source of illness despite the use of a variety of agents to treat them.
Objectives: To assess the effectiveness of interventions for treating oral mucositis or its associated pain in patients with cancer receiving chemotherapy or radiotherapy or both.
Search strategy: Electronic searches of Cochrane Oral Health Group and PaPaS Trials Registers (to 1 June 2010), CENTRAL via The Cochrane Library (to Issue 2, 2010), MEDLINE via OVID (1950 to 1 June 2010), EMBASE via OVID (1980 to 1 June 2010), CINAHL via EBSCO (1980 to 1 June 2010), CANCERLIT via PubMed (1950 to 1 June 2010), OpenSIGLE (1980 to 1 June 2010) and LILACS via the Virtual Health Library (1980 to 1 June 2010) were undertaken. Reference lists from relevant articles were searched and the authors of eligible trials were contacted to identify trials and obtain additional information.
Selection criteria: All randomised controlled trials comparing agents prescribed to treat oral mucositis in people receiving chemotherapy or radiotherapy or both. Outcomes were oral mucositis, time to heal mucositis, oral pain, duration of pain control, dysphagia, systemic infection, amount of analgesia, length of hospitalisation, cost and quality of life.
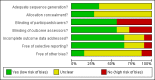
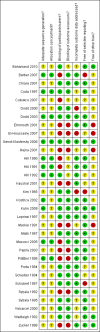
Data collection and analysis: Data were independently extracted, in duplicate, by two review authors. Authors were contacted for details of randomisation, blindness and withdrawals. Risk of bias assessment was carried out on six domains. The Cochrane Collaboration statistical guidelines were followed and risk ratio (RR) values calculated using fixed-effect models (less than 3 trials in each meta-analysis).
Main results: Thirty-two trials involving 1505 patients satisfied the inclusion criteria. Three comparisons for mucositis treatment including two or more trials were: benzydamine HCl versus placebo, sucralfate versus placebo and low level laser versus sham procedure. Only the low level laser showed a reduction in severe mucositis when compared with the sham procedure, RR 5.28 (95% confidence interval (CI) 2.30 to 12.13).Only 3 comparisons included more than one trial for pain control: patient controlled analgesia (PCA) compared to the continuous infusion method, therapist versus control, cognitive behaviour therapy versus control. There was no evidence of a difference in mean pain score between PCA and continuous infusion, however, less opiate was used per hour for PCA, mean difference 0.65 mg/hour (95% CI 0.09 to 1.20), and the duration of pain was less 1.9 days (95% CI 0.3 to 3.5).
Authors' conclusions: There is weak and unreliable evidence that low level laser treatment reduces the severity of the mucositis. Less opiate is used for PCA versus continuous infusion. Further, well designed, placebo or no treatment controlled trials assessing the effectiveness of interventions investigated in this review and new interventions for treating mucositis are needed.
Conflict of interest statement
None known.
Figures








Update of
-
Interventions for treating oral mucositis for patients with cancer receiving treatment.Cochrane Database Syst Rev. 2007 Apr 18;(2):CD001973. doi: 10.1002/14651858.CD001973.pub3. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2010 Aug 04;(8):CD001973. doi: 10.1002/14651858.CD001973.pub4. PMID: 17443514 Updated.
Similar articles
-
Interventions for treating oral mucositis for patients with cancer receiving treatment.Cochrane Database Syst Rev. 2007 Apr 18;(2):CD001973. doi: 10.1002/14651858.CD001973.pub3. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2010 Aug 04;(8):CD001973. doi: 10.1002/14651858.CD001973.pub4. PMID: 17443514 Updated.
-
Interventions for treating oral mucositis for patients with cancer receiving treatment.Cochrane Database Syst Rev. 2004;(2):CD001973. doi: 10.1002/14651858.CD001973.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2007 Apr 18;(2):CD001973. doi: 10.1002/14651858.CD001973.pub3. PMID: 15106165 Updated.
-
Interventions for treating oral mucositis for patients with cancer receiving treatment.Cochrane Database Syst Rev. 2002;(1):CD001973. doi: 10.1002/14651858.CD001973. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2004;(2):CD001973. doi: 10.1002/14651858.CD001973.pub2. PMID: 11869616 Updated.
-
Interventions for preventing oral mucositis for patients with cancer receiving treatment.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD000978. doi: 10.1002/14651858.CD000978.pub3. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2010 Dec 08;(12):CD000978. doi: 10.1002/14651858.CD000978.pub3. PMID: 17943748 Updated.
-
Interventions for preventing oral mucositis for patients with cancer receiving treatment.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD000978. doi: 10.1002/14651858.CD000978.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2007 Oct 17;(4):CD000978. doi: 10.1002/14651858.CD000978.pub3. PMID: 16625538 Updated.
Cited by
-
Is pain part of a systemic syndrome in head and neck cancer?Support Care Cancer. 2020 Feb;28(2):451-459. doi: 10.1007/s00520-019-05147-8. Epub 2019 Nov 12. Support Care Cancer. 2020. PMID: 31713692 Review.
-
Evaluation of topical morphine for treatment of oral mucositis in cancer patients.Br J Pain. 2021 Nov;15(4):411-419. doi: 10.1177/2049463720975061. Epub 2020 Nov 30. Br J Pain. 2021. PMID: 34840789 Free PMC article.
-
A critical assessment of oral care protocols for patients under radiation therapy in the regional University Hospital Network of Madrid (Spain).J Clin Exp Dent. 2015 Dec 1;7(5):e613-21. doi: 10.4317/jced.52557. eCollection 2015 Dec. J Clin Exp Dent. 2015. PMID: 26644838 Free PMC article.
-
Systematic review of cytokines and growth factors for the management of oral mucositis in cancer patients.Support Care Cancer. 2013 Jan;21(1):343-55. doi: 10.1007/s00520-012-1594-5. Epub 2012 Sep 18. Support Care Cancer. 2013. PMID: 22987094
-
Treatment of oral mucositis due to chemotherapy.J Clin Exp Dent. 2016 Apr 1;8(2):e201-9. doi: 10.4317/jced.52917. eCollection 2016 Apr. J Clin Exp Dent. 2016. PMID: 27034762 Free PMC article. Review.
References
References to studies included in this review
-
- Barber C, Powell R, Ellis A, Hewett J. Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: a preliminary, double centre, randomised controlled trial on patients with radiotherapy‐induced oral mucositis. Supportive Care in Cancer 2007;15(4):427‐40. - PubMed
-
- Chiara S, Nobile MT, Vincenti M, Gozza A, Pastrone I, Rosso M, et al. Sucralfate in the treatment of chemotherapy‐induced stomatitis: a double‐blind, placebo‐controlled pilot study. Anticancer Research 2001;21(5):3707‐10. - PubMed
-
- Coda BA, O'Sullivan B, Donaldson G, Bohl S, Chapman CR, Shen DD. Comparative efficacy of patient‐controlled administration of morphine, hydromorphone, or sufentanil for the treatment of oral mucositis pain following bone marrow transplantation. Pain 1997;72(3):333‐46. - PubMed
-
- Cubucku CE, Sevenir B. Debridement could be a solution to promote healing of established mucositis in children. European Archives of Paediatric Dentistry 2007;8(2):105‐12. - PubMed
References to studies excluded from this review
-
- Agüeros P, Bárcena M, Campo M, Lucero P, Bermudez A, Yanez L. Efficacy of vitamin E during acute mucositis in bone marrow transplantation. Bone Marrow Transplantation 2004;33(Suppl 1):S291.
-
- Allison RR, Vongtama V, Vaughan J, Shin KH. Symptomatic acute mucositis can be minimized or prophylaxed by the combination of sucralfate and fluconazole. Cancer Investigations 1995;13(1):16‐22. - PubMed
-
- Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, et al. Randomized phase II study of multiple dose levels of CCI‐779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. Journal of Clinical Oncology 2004;22(5):909‐18. - PubMed
-
- Awada A, Gil T, Sales F, Dubuisson M, Vereecken P, Klastersky J, et al. Prolonged schedule of temozolomide (Temodal) plus liposomal doxorubicin (Caelyx) in advanced solid cancers. Anticancer Drugs 2004;15(5):499‐502. - PubMed
-
- Barker G, Loftus L, Cuddy P, Barker B. The effects of sucralfate suspension and diphenhydramine syrup plus kaolin‐pectin on radiotherapy‐induced mucositis. Oral Surgery, Oral Medicine, Oral Pathology 1991;71(3):288‐93. - PubMed
Additional references
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. - PubMed
-
- Bellm LA, Cunningham G, Durnell L, Eilers J, Epstein JB, Fleming T. Defining clinically meaningful outcomes in the evaluation of new treatments for oral mucositis: oral mucositis patient provider advisory board. Cancer Investigations 2002;20(5‐6):793‐800. - PubMed
-
- Pauw BE. Practical modalities for prevention of fungal infections in cancer patients. European Journal of Clinical Microbiology & Infectious Diseases 1997;16(1):32‐41. - PubMed
-
- Denning DW, Donnelly JP, Hellreigel KP, Ito J, Martino P, van't Wout JW. Antifungal prophylaxis during neutropenia or allogeneic bone marrow transplantation: what is the state of the art? Ad HOC Working Group. Chemotherapy 1992;38 Suppl(1):43‐9. - PubMed
References to other published versions of this review
-
- Worthington HV, Clarkson JE, Eden OB. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database of Systematic Reviews 2004, Issue 2. [Art. No.: CD001973. DOI: 10.1002/14651858.CD001973.pub2] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

