Prolonged survival and phenotypic correction of Akp2(-/-) hypophosphatasia mice by lentiviral gene therapy
- PMID: 20687159
- PMCID: PMC3179312
- DOI: 10.1002/jbmr.201
Prolonged survival and phenotypic correction of Akp2(-/-) hypophosphatasia mice by lentiviral gene therapy
Abstract
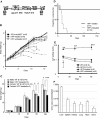
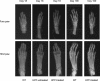
Hypophosphatasia (HPP) is an inherited systemic skeletal disease caused by mutations in the gene encoding the tissue-nonspecific alkaline phosphatase (TNALP) isozyme. The clinical severity of HPP varies widely, with symptoms including rickets and osteomalacia. TNALP knockout (Akp2(-/-)) mice phenotypically mimic the severe infantile form of HPP; that is, TNALP-deficient mice are born with a normal appearance but die by 20 days of age owing to growth failure, hypomineralization, and epileptic seizures. In this study, a lentiviral vector expressing a bone-targeted form of TNALP was injected into the jugular vein of newborn Akp2(-/-) mice. We found that alkaline phosphatase activity in the plasma of treated Akp2(-/-) mice increased and remained at high levels throughout the life of the animals. The treated Akp2(-/-) mice survived for more than 10 months and demonstrated normal physical activity and a healthy appearance. Epileptic seizures were completely inhibited in the treated Akp2(-/-) mice, and X-ray examination of the skeleton showed that mineralization was significantly improved by the gene therapy. These results show that severe infantile HPP in TNALP knockout mice can be treated with a single injection of lentiviral vector during the neonatal period.
© 2011 American Society for Bone and Mineral Research.
Figures

References
-
- Rathbun JC. Hypophosphatasia: a new developmental anomaly. Am J Dis Child. 1948;75:822–831. - PubMed
-
- Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994;15:439–461. - PubMed
-
- Whyte MP. Hypophosphataia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, editors. The Metabolic and Molecular Bases of Inherited Deseases. 8th ed. IV. New York: McGraw-Hill; 2002. pp. 5319–5329.
-
- Pimstone B, Eisenberg E, Silverman S. Hypophosphatasia: genetic and dental studies. Ann Intern Med. 1966;65:722–7229. - PubMed
-
- Seetharam B, Tiruppathi C, Alpers DH. Hydrophobic interactions of brush border alkaline phosphatases: the role of phosphatidyl inositol. Arch Biochem Biophys. 1987;253:189–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

