Tyrosine 112 is essential for organic cation transport by the plasma membrane monoamine transporter
- PMID: 20687515
- PMCID: PMC2995015
- DOI: 10.1021/bi100560q
Tyrosine 112 is essential for organic cation transport by the plasma membrane monoamine transporter
Abstract
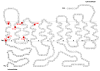
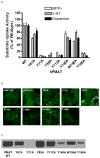
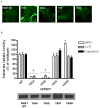
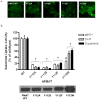
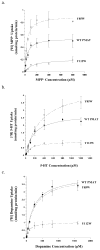
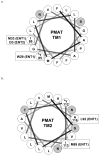
Plasma membrane monoamine transporter (PMAT) is a polyspecific organic cation transporter in the solute carrier 29 (SLC29) family. Previous studies suggested that the major substrate recognition sites are located within transmembrane domains (TM) 1-6, and interaction of PMAT with organic cations may involve aromatic residues. In this study, we analyzed the roles of tyrosine and tryptophan residues located within TM1-6 with a goal of identifying potential residues involved in substrate recognition and translocation. The six tyrosines and one tryptophan in this region were each mutated to alanine followed by analysis of the mutant's membrane localization and transport activity toward 1-methyl-4-phenylpyridinium (MPP(+)), serotonin (5-HT), and dopamine. Two mutants, Y85A and Y112A, exhibited normal cell surface expressions but lost their transport activities toward organic cations. At position Y85, aromatic substitution with phenylalanine or tryptophan fully restored organic cation transport activity. Interestingly, at position Y112, phenylalanine substitution was not allowed. Tryptophan substitution at Y112 partially restored transport activity toward 5-HT and dopamine but severely impaired MPP(+) transport. Detailed kinetic analyses revealed that tryptophan substitution at Y85 and Y112 affected the apparent binding affinity (K(m)) and maximal transport velocity (V(max)) in a substrate-dependent manner. Together, our data suggest that Y85 and Y112 are important molecular determinants for PMAT function, and Y112 is indispensable for optimal interaction with organic cation substrates. Our analyses also suggest the involvement of transmembrane domains 1 and 2 in forming the substrate permeation pathway of PMAT.
Figures

Similar articles
-
Molecular determinants of substrate selectivity of a novel organic cation transporter (PMAT) in the SLC29 family.J Biol Chem. 2007 Feb 2;282(5):3188-95. doi: 10.1074/jbc.M609421200. Epub 2006 Nov 22. J Biol Chem. 2007. PMID: 17121826 Free PMC article.
-
Adenosine transport by plasma membrane monoamine transporter: reinvestigation and comparison with organic cations.Drug Metab Dispos. 2010 Oct;38(10):1798-805. doi: 10.1124/dmd.110.032987. Epub 2010 Jun 30. Drug Metab Dispos. 2010. PMID: 20592246 Free PMC article.
-
Residue Ile89 in human plasma membrane monoamine transporter influences its organic cation transport activity and sensitivity to inhibition by dilazep.Biochem Pharmacol. 2012 Aug 1;84(3):383-90. doi: 10.1016/j.bcp.2012.04.018. Epub 2012 May 4. Biochem Pharmacol. 2012. PMID: 22562044 Free PMC article.
-
Histamine Clearance Through Polyspecific Transporters in the Brain.Handb Exp Pharmacol. 2017;241:173-187. doi: 10.1007/164_2016_13. Handb Exp Pharmacol. 2017. PMID: 27679412 Review.
-
[Research progress of pyrilamine-sensitive H~+/OC antiporter].Yao Xue Xue Bao. 2016 Jun;51(6):886-91. Yao Xue Xue Bao. 2016. PMID: 29878742 Review. Chinese.
Cited by
-
Molecular analysis and structure-activity relationship modeling of the substrate/inhibitor interaction site of plasma membrane monoamine transporter.J Pharmacol Exp Ther. 2011 Nov;339(2):376-85. doi: 10.1124/jpet.111.184036. Epub 2011 Aug 4. J Pharmacol Exp Ther. 2011. PMID: 21816955 Free PMC article.
-
Brain Plasma Membrane Monoamine Transporter in Health and Disease.Handb Exp Pharmacol. 2021;266:253-280. doi: 10.1007/164_2021_446. Handb Exp Pharmacol. 2021. PMID: 33751232
-
Substituted-cysteine accessibility and cross-linking identify an exofacial cleft in the 7th and 8th helices of the proton-coupled folate transporter (SLC46A1).Am J Physiol Cell Physiol. 2018 Mar 1;314(3):C289-C296. doi: 10.1152/ajpcell.00215.2017. Epub 2017 Nov 22. Am J Physiol Cell Physiol. 2018. PMID: 29167151 Free PMC article.
-
The plasma membrane monoamine transporter (PMAT): Structure, function, and role in organic cation disposition.Clin Pharmacol Ther. 2016 Nov;100(5):489-499. doi: 10.1002/cpt.442. Epub 2016 Sep 19. Clin Pharmacol Ther. 2016. PMID: 27506881 Free PMC article. Review.
-
Interspecies comparison of the functional characteristics of plasma membrane monoamine transporter (PMAT) between human, rat and mouse.J Chem Neuroanat. 2017 Oct;83-84:99-106. doi: 10.1016/j.jchemneu.2016.09.006. Epub 2016 Sep 15. J Chem Neuroanat. 2017. PMID: 27641077 Free PMC article.
References
-
- Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5:63–84. - PubMed
-
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. - PubMed
-
- Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, Ritzel MW, Cass CE, Young JD. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem. 2005;280:15880–15887. - PubMed
-
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–50049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

