Noncovalent interaction of alpha(2)-antiplasmin with fibrin(ogen): localization of alpha(2)-antiplasmin-binding sites
- PMID: 20687529
- PMCID: PMC2932838
- DOI: 10.1021/bi1010317
Noncovalent interaction of alpha(2)-antiplasmin with fibrin(ogen): localization of alpha(2)-antiplasmin-binding sites
Abstract
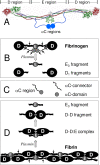
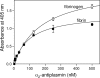
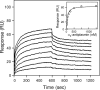
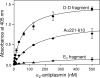
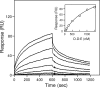
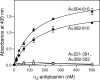
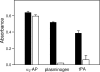
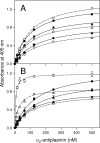
Covalent incorporation (cross-linking) of plasmin inhibitor alpha(2)-antiplasmin (alpha(2)-AP) into fibrin clots increases their resistance to fibrinolysis. We hypothesized that alpha(2)-AP may also interact noncovalently with fibrin prior to its covalent cross-linking. To test this hypothesis, we studied binding of alpha(2)-AP to fibrin(ogen) and its fragments by an enzyme-linked immunosorbent assay (ELISA) and surface plasmon resonance. The experiments revealed that alpha(2)-AP binds to polymeric fibrin and surface-adsorbed fibrin(ogen), while no binding was observed with fibrinogen in solution. To localize the alpha(2)-AP-binding sites, we studied the interaction of alpha(2)-AP with the fibrin(ogen)-derived D(1), D-D, and E(3) fragments, and the recombinant alphaC region and its constituents, alphaC connector and alphaC domain and its subdomains, which together encompass practically the whole fibrin(ogen) molecule. In the ELISA, alpha(2)-AP bound to immobilized D(1), D-D, alphaC region, alphaC domain, and its C-terminal subdomain. The binding was Lys-independent and was not inhibited by plasminogen or tPA. Furthermore, the affinity of alpha(2)-AP for D-D was significantly increased in the presence of plasminogen, while that to the alphaC domain remained unaffected. Altogether, these results indicate that the fibrin(ogen) D region and the C-terminal subdomain of the alphaC domain contain high-affinity alpha(2)-AP-binding sites that are cryptic in fibrinogen and exposed in fibrin or adsorbed fibrinogen, and the presence of plasminogen facilitates interaction of alpha(2)-AP with the D regions. The discovered noncovalent interaction of alpha(2)-AP with fibrin may contribute to regulation of the initial stage of fibrinolysis and provide proper orientation of the cross-linking sites to facilitate covalent cross-linking of alpha(2)-AP to the fibrin clot.
Figures

Similar articles
-
Interaction of fibrin(ogen) with fibronectin: further characterization and localization of the fibronectin-binding site.Biochemistry. 2002 Jun 25;41(25):7907-13. doi: 10.1021/bi025770x. Biochemistry. 2002. PMID: 12069579
-
Cross-linking of plasminogen activator inhibitor 2 and alpha 2-antiplasmin to fibrin(ogen).J Biol Chem. 2000 Aug 11;275(32):24915-20. doi: 10.1074/jbc.M002901200. J Biol Chem. 2000. PMID: 10816585
-
Identification and characterization of novel lysine-independent apolipoprotein(a)-binding sites in fibrin(ogen) alphaC-domains.J Biol Chem. 2003 Sep 26;278(39):37154-9. doi: 10.1074/jbc.M305154200. Epub 2003 Jul 9. J Biol Chem. 2003. PMID: 12853452
-
Molecular mechanisms of initiation of fibrinolysis by fibrin.Thromb Haemost. 2003 Mar;89(3):409-19. Thromb Haemost. 2003. PMID: 12624622 Review.
-
Fibrinogen and fibrin structure and functions.J Thromb Haemost. 2005 Aug;3(8):1894-904. doi: 10.1111/j.1538-7836.2005.01365.x. J Thromb Haemost. 2005. PMID: 16102057 Review.
Cited by
-
Fibrin Formation, Structure and Properties.Subcell Biochem. 2017;82:405-456. doi: 10.1007/978-3-319-49674-0_13. Subcell Biochem. 2017. PMID: 28101869 Free PMC article. Review.
-
Surface-bound FXIII enhances deposition and straightness of fibrin fibers.Biophys Rep (N Y). 2025 Jun 11;5(2):100207. doi: 10.1016/j.bpr.2025.100207. Epub 2025 Mar 24. Biophys Rep (N Y). 2025. PMID: 40139391 Free PMC article.
-
A targeted LC MS/MS assay of a health surveillance panel and its application to chronic kidney disease.bioRxiv [Preprint]. 2025 Mar 16:2025.03.14.643399. doi: 10.1101/2025.03.14.643399. bioRxiv. 2025. PMID: 40161722 Free PMC article. Preprint.
-
Transglutaminase Activities of Blood Coagulant Factor XIII Are Dependent on the Activation Pathways and on the Substrates.Thromb Haemost. 2023 Apr;123(4):380-392. doi: 10.1055/a-1993-4193. Epub 2022 Dec 6. Thromb Haemost. 2023. PMID: 36473493 Free PMC article.
-
GPVI (Glycoprotein VI) Interaction With Fibrinogen Is Mediated by Avidity and the Fibrinogen αC-Region.Arterioscler Thromb Vasc Biol. 2021 Mar;41(3):1092-1104. doi: 10.1161/ATVBAHA.120.315030. Epub 2021 Jan 21. Arterioscler Thromb Vasc Biol. 2021. PMID: 33472402 Free PMC article.
References
-
- Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb. Haemost. 2001;86:324–333. - PubMed
-
- Lijnen HR. Elements of the fibrinolytic system. Ann. N.Y. Acad. Sci. 2001;936:226–236. - PubMed
-
- Rijken DC, Lijnen HR. New insights into the molecular mechanisms of the fibrinolytic system. J. Thromb. Haemost. 2009;7:4–13. - PubMed
-
- Medved L, Nieuwenhuizen W. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb. Haemost. 2003;89:409–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

