Bovine serum albumin-catalyzed deprotonation of [1-(13)C]glycolaldehyde: protein reactivity toward deprotonation of the alpha-hydroxy alpha-carbonyl carbon
- PMID: 20687575
- PMCID: PMC2932853
- DOI: 10.1021/bi101118g
Bovine serum albumin-catalyzed deprotonation of [1-(13)C]glycolaldehyde: protein reactivity toward deprotonation of the alpha-hydroxy alpha-carbonyl carbon
Abstract
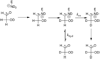
Bovine serum albumin (BSA) in D(2)O at 25 degrees C and pD 7.0 was found to catalyze the deuterium exchange reactions of [1-(13)C]glycolaldehyde ([1-(13)C]GA) to form [1-(13)C,2-(2)H]GA and [1-(13)C,2,2-di-(2)H]GA. The formation of [1-(13)C,2-(2)H]GA and [1-(13)C,2,2-di-(2)H]GA in a total yield of 51 +/- 3% was observed at early reaction times, and at later times, [1-(13)C,2-(2)H]GA was found to undergo BSA-catalyzed conversion to [1-(13)C,2,2-di-(2)H]GA. The overall second-order rate constant for these deuterium exchange reactions [(k(E))(P)] equals 0.25 M(-1) s(-1). By comparison, (k(E))(P) values of 0.04 M(-1) s(-1) [Go, M. K., Amyes, T. L., and Richard, J. P. (2009) Biochemistry 48, 5769-5778] and 0.06 M(-1) s(-1) [Go, M. K., Koudelka, A., Amyes, T. L., and Richard, J. P. (2010) Biochemistry 49, 5377-5389] have been determined for the wild-type- and K12G mutant TIM-catalyzed deuterium exchange reactions of [1-(13)C]GA, respectively, to form [1-(13)C,2,2-di-(2)H]GA. These data show that TIM and BSA exhibit a modest catalytic activity toward deprotonation of the alpha-hydroxy alpha-carbonyl carbon. We suggest that this activity is intrinsic to many globular proteins, and that it must be enhanced to demonstrate meaningful de novo design of protein catalysts of proton transfer at alpha-carbonyl carbon.
Figures




References
-
- Hall A, Knowles JR. The uncatalyzed rates of enolization of dihydroxyacetone phoshate and of glyceraldehyde 3-phosphate in neutral aqueous solution. The quantitative assessment of the effectiveness of an enzyme catalyst. Biochemistry. 1975;14:4348–4353. - PubMed
-
- Richard JP. Acid-base catalysis of the elimination and isomerization reactions of triose phosphates. J. Am. Chem. Soc. 1984;106:4926–4936.
-
- Hartman FC. Haloacetol phosphates. Characterization of the active site of rabbit muscle triose phosphate isomerase. Biochemistry. 1971;10:146–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

