Total synthesis of fostriecin: via a regio- and stereoselective polyene hydration, oxidation, and hydroboration sequence
- PMID: 20687585
- PMCID: PMC2929272
- DOI: 10.1021/ol101340n
Total synthesis of fostriecin: via a regio- and stereoselective polyene hydration, oxidation, and hydroboration sequence
Abstract
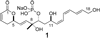
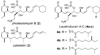
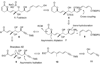
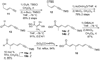
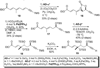
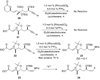
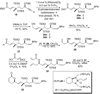
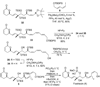
A total synthesis of the fostriecin has been achieved in 24 steps from enyne 11. The lactone moiety was installed by a Leighton allylation and Grubbs ring-closing metathesis reaction. The highly reactive Z,Z,E-triene moiety was installed via a late-stage Suzuki-Miyaura cross-coupling of a remarkably stable Z-vinyl boronate. The relative and absolute stereocenters of the C-8,9,11 triol were generated with a regio- and stereoselective asymmetric hydration/oxidation sequence.
Figures
References
-
-
Reviews: Jackson RC, Fry DW, Boritzki TJ, Roberts BJ, Hook KE, Leopold WR. Adv. Enzyme Regul. 1985;23:193. Scheithauer W, Hoff DDV, Clark GM, Shillis JL, Elslager EF. Eur. J. Clin. Oncol. 1986;22:921.
-
-
-
Reviews: Walsh AH, Cheng A, Honkanen RE. FEBS Lett. 1997;416:230. Hastie CJ, Cohen PTW. FEBS Lett. 1998;431:357.
-
-
- Boger DL, Hikota M, Lewis BM. J. Org. Chem. 1997;62:1748.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

