Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1
- PMID: 20687910
- PMCID: PMC2922194
- DOI: 10.1186/1476-4598-9-209
Tumor cell-derived PDGF-B potentiates mouse mesenchymal stem cells-pericytes transition and recruitment through an interaction with NRP-1
Abstract
Background: New blood vessel formation, or angiogenic switch, is an essential event in the development of solid tumors and their metastatic growth. Tumor blood vessel formation and remodeling is a complex and multi-step processes. The differentiation and recruitment of mural cells including vascular smooth muscle cells and pericytes are essential steps in tumor angiogenesis. However, the role of tumor cells in differentiation and recruitment of mural cells has not yet been fully elucidated. This study focuses on the role of human tumor cells in governing the differentiation of mouse mesenchymal stem cells (MSCs) to pericytes and their recruitment in the tumor angiogenesis process.
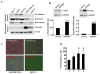
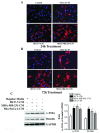
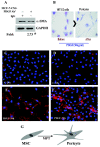
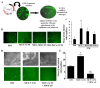
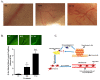
Results: We show that C3H/10T1/2 mouse embryonic mesenchymal stem cells, under the influence of different tumor cell-derived conditioned media, differentiate into mature pericytes. These differentiated pericytes, in turn, are recruited to bind with capillary-like networks formed by endothelial cells on the matrigel under in vitro conditions and recruited to bind with blood vessels on gel-foam under in vivo conditions. The degree of recruitment of pericytes into in vitro neo-angiogenesis is tumor cell phenotype specific. Interestingly, invasive cells recruit less pericytes as compared to non-invasive cells. We identified tumor cell-secreted platelet-derived growth factor-B (PDGF-B) as a crucial factor controlling the differentiation and recruitment processes through an interaction with neuropilin-1 (NRP-1) in mesenchymal stem cells.
Conclusion: These new insights into the roles of tumor cell-secreted PDGF-B-NRP-1 signaling in MSCs-fate determination may help to develop new antiangiogenic strategies to prevent the tumor growth and metastasis and result in more effective cancer therapies.
Figures
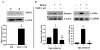
Similar articles
-
Human Glioblastoma-Derived Mesenchymal Stem Cell to Pericytes Transition and Angiogenic Capacity in Glioblastoma Microenvironment.Cell Physiol Biochem. 2018;46(1):279-290. doi: 10.1159/000488429. Epub 2018 Mar 22. Cell Physiol Biochem. 2018. PMID: 29590646
-
Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse.Development. 1999 Jun;126(14):3047-55. doi: 10.1242/dev.126.14.3047. Development. 1999. PMID: 10375497
-
Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors.J Clin Invest. 2003 Oct;112(8):1142-51. doi: 10.1172/JCI18549. J Clin Invest. 2003. PMID: 14561699 Free PMC article.
-
Role of pericytes in vascular morphogenesis.EXS. 2005;(94):115-25. doi: 10.1007/3-7643-7311-3_8. EXS. 2005. PMID: 15617474 Review.
-
Defining vascular stem cells.Stem Cells Dev. 2013 Apr 1;22(7):1018-26. doi: 10.1089/scd.2012.0504. Epub 2013 Jan 18. Stem Cells Dev. 2013. PMID: 23330734 Free PMC article. Review.
Cited by
-
The potential for resident lung mesenchymal stem cells to promote functional tissue regeneration: understanding microenvironmental cues.Cells. 2012 Dec;1(4):874. doi: 10.3390/cells1040874. Cells. 2012. PMID: 23626909 Free PMC article.
-
Biogenesis, Composition and Potential Therapeutic Applications of Mesenchymal Stem Cells Derived Exosomes in Various Diseases.Int J Nanomedicine. 2023 Jun 14;18:3177-3210. doi: 10.2147/IJN.S407029. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37337578 Free PMC article. Review.
-
Tumor-associated mesenchymal stem/stromal cells in tumor microenvironment and carcinogenesis.Exp Hematol Oncol. 2025 Jul 17;14(1):97. doi: 10.1186/s40164-025-00688-7. Exp Hematol Oncol. 2025. PMID: 40676710 Free PMC article. Review.
-
Metformin-dependent variation of microglia phenotype dictates pericytes maturation under oxygen-glucose deprivation.Tissue Barriers. 2022 Oct 2;10(4):2018928. doi: 10.1080/21688370.2021.2018928. Epub 2022 Jan 5. Tissue Barriers. 2022. PMID: 34983297 Free PMC article.
-
Tumor angiogenesis: pericytes and maturation are not to be ignored.J Oncol. 2012;2012:261750. doi: 10.1155/2012/261750. Epub 2011 Oct 9. J Oncol. 2012. PMID: 22007214 Free PMC article.
References
-
- Folkman J. Angiogenesis and angiogenesis inhibition: an overview. EXS. 1997;79:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

