Brg-1 mediates the constitutive and fenretinide-induced expression of SPARC in mammary carcinoma cells via its interaction with transcription factor Sp1
- PMID: 20687958
- PMCID: PMC2924311
- DOI: 10.1186/1476-4598-9-210
Brg-1 mediates the constitutive and fenretinide-induced expression of SPARC in mammary carcinoma cells via its interaction with transcription factor Sp1
Abstract
Background: Secreted protein, acidic and rich in cysteine (SPARC) is a matricellular protein that mediates cell-matrix interactions. It has been shown, depending on the type of cancer, to possess either pro- or anti-tumorigenic properties. The transcriptional regulation of the SPARC gene expression has not been fully elucidated and the effects of anti-cancer drugs on this process have not been explored.
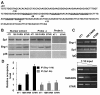
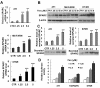
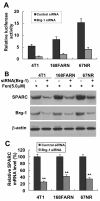
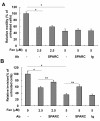
Results: In the present study, we demonstrated that chromatin remodeling factor Brg-1 is recruited to the proximal SPARC promoter region (-130/-56) through an interaction with transcription factor Sp1. We identified Brg-1 as a critical regulator for the constitutive expression levels of SPARC mRNA and protein in mammary carcinoma cell lines and for SPARC secretion into culture media. Furthermore, we found that Brg-1 cooperates with Sp1 to enhance SPARC promoter activity. Interestingly, fenretinide [N-4(hydroxyphenyl) retinamide, 4-HPR], a synthetic retinoid with anti-cancer properties, was found to up-regulate the transcription, expression and secretion of SPARC via induction of the Brg-1 in a dose-dependent manner. Finally, our results demonstrated that fenretinide-induced expression of SPARC contributes significantly to a decreased invasion of mammary carcinoma cells.
Conclusions: Overall, our results reveal a novel cooperative role of Brg-1 and Sp1 in mediating the constitutive and fenretinide-induced expression of SPARC, and provide new insights for the understanding of the anti-cancer effects of fenretinide.
Figures
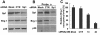


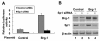


Similar articles
-
Transcriptional upregulation of SPARC, in response to c-Jun overexpression, contributes to increased motility and invasion of MCF7 breast cancer cells.Oncogene. 2002 Oct 10;21(46):7077-91. doi: 10.1038/sj.onc.1205857. Oncogene. 2002. PMID: 12370830
-
Brg-1 is required for maximal transcription of the human matrix metalloproteinase-2 gene.J Biol Chem. 2004 Oct 29;279(44):46326-34. doi: 10.1074/jbc.M405438200. Epub 2004 Aug 17. J Biol Chem. 2004. PMID: 15317818
-
v-Jun downregulates the SPARC target gene by binding to the proximal promoter indirectly through Sp1/3.Oncogene. 2003 Jun 26;22(26):4047-61. doi: 10.1038/sj.onc.1206713. Oncogene. 2003. PMID: 12821939
-
Transcription factor SOX-5 enhances nasopharyngeal carcinoma progression by down-regulating SPARC gene expression.J Pathol. 2008 Mar;214(4):445-55. doi: 10.1002/path.2299. J Pathol. 2008. PMID: 18085523
-
A 9-kDa matricellular SPARC fragment released by cathepsin D exhibits pro-tumor activity in the triple-negative breast cancer microenvironment.Theranostics. 2021 Apr 15;11(13):6173-6192. doi: 10.7150/thno.58254. eCollection 2021. Theranostics. 2021. PMID: 33995652 Free PMC article.
Cited by
-
HDAC10 inhibition represses melanoma cell growth and BRAF inhibitor resistance via upregulating SPARC expression.NAR Cancer. 2024 Apr 22;6(2):zcae018. doi: 10.1093/narcan/zcae018. eCollection 2024 Jun. NAR Cancer. 2024. PMID: 38650694 Free PMC article.
-
Global O-GlcNAc Levels Modulate Transcription of the Adipocyte Secretome during Chronic Insulin Resistance.Front Endocrinol (Lausanne). 2015 Jan 22;5:223. doi: 10.3389/fendo.2014.00223. eCollection 2014. Front Endocrinol (Lausanne). 2015. PMID: 25657638 Free PMC article.
-
Pten regulates collagen fibrillogenesis by fibroblasts through SPARC.PLoS One. 2021 Feb 3;16(2):e0245653. doi: 10.1371/journal.pone.0245653. eCollection 2021. PLoS One. 2021. PMID: 33534863 Free PMC article.
-
Sp1 facilitates DNA double-strand break repair through a nontranscriptional mechanism.Mol Cell Biol. 2012 Sep;32(18):3790-9. doi: 10.1128/MCB.00049-12. Epub 2012 Jul 23. Mol Cell Biol. 2012. PMID: 22826432 Free PMC article.
References
-
- Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

