High-throughput automated image analysis of neuroinflammation and neurodegeneration enables quantitative assessment of virus neurovirulence
- PMID: 20688036
- PMCID: PMC2997939
- DOI: 10.1016/j.vaccine.2010.07.070
High-throughput automated image analysis of neuroinflammation and neurodegeneration enables quantitative assessment of virus neurovirulence
Abstract
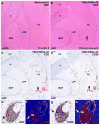
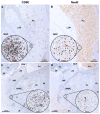
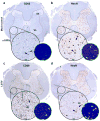
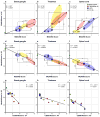
Historically, the safety of live attenuated vaccine candidates against neurotropic viruses was assessed by semi-quantitative analysis of virus-induced histopathology in the central nervous system of monkeys. We have developed a high-throughput automated image analysis (AIA) for the quantitative assessment of virus-induced neuroinflammation and neurodegeneration. Evaluation of the results generated by AIA showed that quantitative estimates of lymphocytic infiltration, microglial activation, and neurodegeneration strongly and significantly correlated with results of traditional histopathological scoring. In addition, we show that AIA is a targeted, objective, accurate, and time-efficient approach that provides reliable differentiation of virus neurovirulence. As such, it may become a useful tool in establishing consistent analytical standards across research and development laboratories and regulatory agencies, and may improve the safety evaluation of live virus vaccines. The implementation of this high-throughput AIA will markedly advance many fields of research including virology, neuroinflammation, neuroscience, and vaccinology.
Published by Elsevier Ltd.
Conflict of interest statement
Figures


Similar articles
-
Safety evaluation of chimeric Langat/Dengue 4 flavivirus, a live vaccine candidate against tick-borne encephalitis.J Med Virol. 2009 Oct;81(10):1777-85. doi: 10.1002/jmv.21587. J Med Virol. 2009. PMID: 19697399
-
Comparison of live and inactivated tick-borne encephalitis virus vaccines for safety, immunogenicity and efficacy in rhesus monkeys.Vaccine. 2006 Jan 12;24(2):133-43. doi: 10.1016/j.vaccine.2005.07.067. Epub 2005 Aug 9. Vaccine. 2006. PMID: 16115704
-
Insertion of microRNA targets into the flavivirus genome alters its highly neurovirulent phenotype.J Virol. 2011 Feb;85(4):1464-72. doi: 10.1128/JVI.02091-10. Epub 2010 Dec 1. J Virol. 2011. PMID: 21123372 Free PMC article.
-
Microfluidics for High-Throughput Quantitative Studies of Early Development.Annu Rev Biomed Eng. 2016 Jul 11;18:285-309. doi: 10.1146/annurev-bioeng-100515-013926. Epub 2016 Feb 29. Annu Rev Biomed Eng. 2016. PMID: 26928208 Review.
-
Automated image analysis for high-content screening and analysis.J Biomol Screen. 2010 Aug;15(7):726-34. doi: 10.1177/1087057110370894. Epub 2010 May 20. J Biomol Screen. 2010. PMID: 20488979 Review.
Cited by
-
Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis.Mol Autism. 2011 May 16;2(1):7. doi: 10.1186/2040-2392-2-7. Mol Autism. 2011. PMID: 21575186 Free PMC article.
-
Spleen tyrosine kinase (SYK) blocks autophagic Tau degradation in vitro and in vivo.J Biol Chem. 2019 Sep 6;294(36):13378-13395. doi: 10.1074/jbc.RA119.008033. Epub 2019 Jul 19. J Biol Chem. 2019. PMID: 31324720 Free PMC article.
-
Targeted deletion of the gene encoding the La autoantigen (Sjögren's syndrome antigen B) in B cells or the frontal brain causes extensive tissue loss.Mol Cell Biol. 2014 Jan;34(1):123-31. doi: 10.1128/MCB.01010-13. Epub 2013 Nov 4. Mol Cell Biol. 2014. PMID: 24190965 Free PMC article.
-
Suppression of the motor deficit in a mucolipidosis type IV mouse model by bone marrow transplantation.Hum Mol Genet. 2016 Jul 1;25(13):2752-2761. doi: 10.1093/hmg/ddw132. Epub 2016 Jun 7. Hum Mol Genet. 2016. PMID: 27270598 Free PMC article.
References
-
- Afzal MA, Marsden SA, Hull RM, Pipkin PA, Bentley ML, Minor PD. Evaluation of the neurovirulence test for mumps vaccines. Biologicals. 1999;27:43–9. - PubMed
-
- Rubin SA, Snoy PJ, Wright KE, Brown EG, Reeve P, Beeler JA, et al. The mumps virus neurovirulence safety test in Rhesus monkeys: a comparison of mumps virus strains. J Infect Dis. 1999;180:521–5. - PubMed
-
- WHO. Final report. IABS Scientific Workshop on Neurovirulence Tests for Live Virus Vaccines. Geneva, Switzerland: WHO; 2005. pp. 1–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

