Ischemic diabetic retinopathy may protect against nuclear sclerotic cataract
- PMID: 20688316
- PMCID: PMC2945444
- DOI: 10.1016/j.ajo.2010.05.013
Ischemic diabetic retinopathy may protect against nuclear sclerotic cataract
Abstract
Purpose: To determine whether diabetes mellitus is protective for nuclear sclerotic cataract at baseline and 6 and 12 months after vitrectomy surgery.
Design: Prospective, interventional cohort study.
Methods: Phakic diabetic and nondiabetic patients undergoing vitrectomy surgery for a variety of retinal conditions underwent Scheimpflug lens photography in the operated and fellow eye at baseline and at 6 and 12 months after vitrectomy surgery.
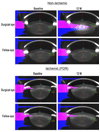
Results: Of 52 eyes included in the analysis, 23 eyes were from diabetic patients, 14 of which had surgery for ischemic retinopathy. At baseline, eyes with ischemic diabetic retinopathy had less nuclear sclerotic cataract than nonischemic diabetic and nondiabetic eyes. This was true for eyes undergoing vitrectomy surgery (P = .0001) and for fellow eyes (P = .003). Nuclear sclerotic cataract developed after vitrectomy surgery in nonischemic diabetic eyes and nondiabetic eyes at the same rate. Diabetic eyes with ischemic retinopathy showed no significant progression of nuclear opacification, and therefore had significantly less postvitrectomy nuclear cataract at 6 months (P < 1 × 10(-6)) and at 12 months (P < .001) than nondiabetic or nonischemic diabetic eyes. Normalizing to baseline opacity and adjusting for age and other comorbidities did not alter this result.
Conclusions: Ischemic diabetic retinopathy, not just systemic diabetes mellitus, protected against nuclear sclerotic cataract at baseline and after vitrectomy surgery. These findings are consistent with the hypothesis that increased exposure to oxygen is responsible for nuclear cataract formation.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Small-gauge vitrectomy does not protect against nuclear sclerotic cataract.Retina. 2012 Mar;32(3):499-505. doi: 10.1097/IAE.0b013e31822529cf. Retina. 2012. PMID: 22392091
-
The effect of pars plana vitrectomy and nuclear cataract on oxygen saturation in retinal vessels, diabetic and non-diabetic patients compared.Acta Ophthalmol. 2016 Feb;94(1):41-7. doi: 10.1111/aos.12828. Epub 2015 Aug 27. Acta Ophthalmol. 2016. PMID: 26310901
-
Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract.Am J Ophthalmol. 2006 Jun;141(6):1027-32. doi: 10.1016/j.ajo.2006.01.016. Am J Ophthalmol. 2006. PMID: 16765670
-
Diabetic vitrectomy: the influence of lens status upon surgical outcomes.Curr Opin Ophthalmol. 2008 May;19(3):243-7. doi: 10.1097/ICU.0b013e3282fc25f3. Curr Opin Ophthalmol. 2008. PMID: 18408501 Review.
-
[Is vitrectomy cataractogenic? Study of changes of the crystalline lens after surgery of retinal detachment].J Fr Ophtalmol. 1992;15(8-9):449-54. J Fr Ophtalmol. 1992. PMID: 1294607 Review. French.
Cited by
-
Vitreoretinal influences on lens function and cataract.Philos Trans R Soc Lond B Biol Sci. 2011 Apr 27;366(1568):1293-300. doi: 10.1098/rstb.2010.0228. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21402587 Free PMC article. Review.
-
When less is more: the role of non-vitrectomized vitreous surgery in retinal diseases.Int J Retina Vitreous. 2025 Aug 1;11(1):90. doi: 10.1186/s40942-025-00686-1. Int J Retina Vitreous. 2025. PMID: 40751226 Free PMC article. Review.
-
Predicting speed of progression of lens opacification after pars plana vitrectomy with silicone oil.PLoS One. 2022 May 20;17(5):e0268377. doi: 10.1371/journal.pone.0268377. eCollection 2022. PLoS One. 2022. PMID: 35594273 Free PMC article.
-
Refractive changes after lens-sparing vitrectomy for macular hole and epiretinal membrane.Clin Ophthalmol. 2017 Aug 21;11:1527-1532. doi: 10.2147/OPTH.S141771. eCollection 2017. Clin Ophthalmol. 2017. PMID: 28860703 Free PMC article.
-
Comment Re: Yu AL, Brummeisl W, Schaumberger M, Kampik A, Welge-Lussen U (2010) Vitrectomy does not increase the risk of open-angle glaucoma or ocular hypertension - a 5-year follow-up. Graefes Arch Clin Exp Ophthalmol 248:1407-1414.Graefes Arch Clin Exp Ophthalmol. 2012 Mar;250(3):461-2. doi: 10.1007/s00417-011-1634-8. Epub 2011 Apr 8. Graefes Arch Clin Exp Ophthalmol. 2012. PMID: 21476066 No abstract available.
References
-
- Cook CA, Koretz JF, Pfahnl A, Hyun J, Kaufman PL. Aging of the human crystalline lens and anterior segment. Vision Res. 1994;34(22):2945–2954. - PubMed
-
- Heys KR, Cram SL, Truscott RJ. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004;10:956–963. - PubMed
-
- Tabandeh H, Wilkins M, Thompson G, Nassiri D, Karim A. Hardness and ultrasonic characteristics of the human crystalline lens. J Cat & Ref Surg. 2000;26(6):838–841. - PubMed
-
- Salm M, Belsky D, Sloan FA. Trends in Cost of Major Eye Diseases to Medicare, 1991 to 2000. Am J of Ophthalmol. 2006;142(6):976–982. - PubMed
-
- West S. Epidemiology of Cataract: Accomplishments over 25 years and Future Directions. Ophthalmic Epidemiol. 2007;14(4):173–178. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

