Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling
- PMID: 20688931
- PMCID: PMC3013545
- DOI: 10.1681/ASN.2010030295
Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling
Erratum in
- J Am Soc Nephrol. 2011 Jul;22(7):1390
Abstract
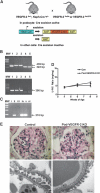
VEGF is a potent vascular growth factor produced by podocytes in the developing and mature glomerulus. Specific deletion of VEGF from podocytes causes glomerular abnormalities including profound endothelial cell injury, suggesting that paracrine signaling is critical for maintaining the glomerular filtration barrier (GFB). However, it is not clear whether normal GFB function also requires autocrine VEGF signaling in podocytes. In this study, we sought to determine whether an autocrine VEGF-VEGFR-2 loop in podocytes contributes to the maintenance of the GFB in vivo. We found that induced, whole-body deletion of VEGFR-2 caused marked abnormalities in the kidney and also other tissues, including the heart and liver. By contrast, podocyte-specific deletion of the VEGFR-2 receptor had no effect on glomerular development or function even up to 6 months old. Unlike cell culture models, enhanced expression of VEGF by podocytes in vivo caused foot process fusion and alterations in slit diaphragm-associated proteins; however, inhibition of VEGFR-2 could not rescue this defect. Although VEGFR-2 was dispensable in the podocyte, glomerular endothelial cells depended on VEGFR-2 expression: postnatal deletion of the receptor resulted in global defects in the glomerular microvasculature. Taken together, our results provide strong evidence for dominant actions of a paracrine VEGF-VEGFR-2 signaling loop both in the developing and in the filtering glomerulus. VEGF produced by the podocyte regulates the structure and function of the adjacent endothelial cell.
Figures






Comment in
-
VEGF receptors and glomerular function.J Am Soc Nephrol. 2010 Oct;21(10):1599-600. doi: 10.1681/ASN.2010080871. Epub 2010 Sep 16. J Am Soc Nephrol. 2010. PMID: 20847145 No abstract available.
References
-
- Ferrara N, Henzel WJ: Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161: 851–858, 1989 - PubMed
-
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N: Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309, 1989 - PubMed
-
- Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlondorff D, Cohen CD: Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 18: 1765–1776, 2007 - PubMed
-
- Bortoloso E, Del Prete D, Dalla Vestra M, Gambaro G, Saller A, Antonucci F, Baggio B, Anglani F, Fioretto P: Quantitave and qualitative changes in vascular endothelial growth factor gene expression in glomeruli of patients with type 2 diabetes. Eur J Endocrinol 150: 799–807, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

