Structures and application of oligosaccharides in human milk
- PMID: 20689231
- PMCID: PMC3066539
- DOI: 10.2183/pjab.86.731
Structures and application of oligosaccharides in human milk
Abstract
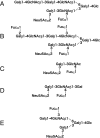
Comparative study of the oligosaccharide profiles of individual human milk revealed the presence of three different patterns. Four oligosaccharides containing the Fucalpha1-2Gal group were missing in the milk of non-secretor, and three oligosaccharides containing the Fucalpha1-4GlcNAc group were missing in the milk of Lewis negative individuals. Disappearance of some major oligosaccharides in these samples led to the finding of five novel minor oligosaccharides, which were hidden under the missing oligosaccharides. Following these studies, structures of many novel milk oligosaccharides were elucidated. At least 13 core oligosaccharides were found in these oligosaccharides. By adding alpha-fucosyl residues and sialic acid residues to these core oligosaccharides, more than one hundred oligosaccharides were formed. All these oligosaccharides contain lactose at their reducing termini. This evidence, together with the deletion phenomena found in the milk oligosaccharides of non-secretor and Lewis negative individuals, suggested that the oligosaccharides are formed from lactose by the concerted action of glycosyltransferases, which are responsible for elongation and branching of the Galbeta1-4GlcNAc group in the sugar chains of glycoconjugates on the surface of epithelial cells. Therefore, oligosaccharides in human milk could include many structures, starting from the Galbeta1-4GlcNAc group in the sugar chains of various glycoconjugates. Many lines of evidence recently indicated that virulent enteric bacteria and viruses start their infection by binding to particular sugar chains of glycoconjugates on the target cell surfaces. Therefore, milk oligosaccharides could be useful for developing drugs, which inhibit the infection of bacteria and viruses.
Figures


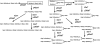
References
-
- Niers L., Stasse-Wolthus M., Rombouts F.M., Rijkers G.T. (2007) Nutritional support for the infant’s immune system. Nutr. Rev. 65, 347–360 - PubMed
-
- Brown C.A., Wang B., Oh J.H. (2008) Antimicrobial activity of lactoferrin against foodborne pathogenic bacteria incorporated into edible chitosan film. J. Food Prot. 71, 319–324 - PubMed
-
- Jenssen H., Hancock R.E. (2009) Antimicrobial properties of lactoferrin. Biochimie 91, 19–29 - PubMed
-
- Polonovski M., Lespagnol A. (1933) Nouvelles acquisitions sur les composés glucidiques du lai de femme. Bull. Soc. Chim. Biol. 15, 320–349
-
- Polonovski M., Montreuil J. (1954) Etude chromatographique des polyosides du lai de femme. C. R. Acad. Sci. 238, 2263–2264 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

