An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia
- PMID: 20689583
- PMCID: PMC2912766
- DOI: 10.1371/journal.pone.0011880
An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia
Erratum in
- PLoS One. 2010;5(9) doi: 10.1371/annotation/73589846-034d-4b6f-9668-54f278fa03e8. Pukittayakamee, Sasithon [corrected to Pukrittayakamee, Sasithon] doi: 10.1371/annotation/73589846-034d-4b6f-9668-54f278fa03e8
- PLoS One. 2010;5(9). doi: 10.1371/annotation/5603e1d7-03ca-4127-ab99-1d1e07d905d4. Dosage error in published abstract; MEDLINE/PubMed abstract corrected doi: 10.1371/annotation/5603e1d7-03ca-4127-ab99-1d1e07d905d4
Abstract
Background: The artemisinin-based combination treatment (ACT) of dihydroartemisinin (DHA) and piperaquine (PQP) is a promising novel anti-malarial drug effective against multi-drug resistant falciparum malaria. The aim of this study was to show non-inferiority of DHA/PQP vs. artesunate-mefloquine (AS+MQ) in Asia.
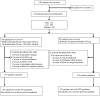
Methods and findings: This was an open-label, randomised, non-inferiority, 63-day follow-up study conducted in Thailand, Laos and India. Patients aged 3 months to 65 years with Plasmodium falciparum mono-infection or mixed infection were randomised with an allocation ratio of 2:1 to a fixed-dose DHA/PQP combination tablet (adults: 40 mg/320 mg; children: 20 mg/160 [DOSAGE ERROR CORRECTED] mg; n = 769) or loose combination of AS+MQ (AS: 50 mg, MQ: 250 mg; n = 381). The cumulative doses of study treatment over the 3 days were of about 6.75 mg/kg of DHA and 54 mg/kg of PQP and about 12 mg/kg of AS and 25 mg/kg of MQ. Doses were rounded up to the nearest half tablet. The primary endpoint was day-63 polymerase chain reaction (PCR) genotype-corrected cure rate. Results were 87.9% for DHA/PQP and 86.6% for AS+MQ in the intention-to-treat (ITT; 97.5% one-sided confidence interval, CI: >-2.87%), and 98.7% and 97.0%, respectively, in the per protocol population (97.5% CI: >-0.39%). No country effect was observed. Kaplan-Meier estimates of proportions of patients with new infections on day 63 (secondary endpoint) were significantly lower for DHA/PQP than AS+MQ: 22.7% versus 30.3% (p = 0.0042; ITT). Overall gametocyte prevalence (days 7 to 63; secondary endpoint), measured as person-gametocyte-weeks, was significantly higher for DHA/PQP than AS+MQ (10.15% versus 4.88%; p = 0.003; ITT). Fifteen serious adverse events were reported, 12 (1.6%) in DHA/PQP and three (0.8%) in AS+MQ, among which six (0.8%) were considered related to DHA/PQP and three (0.8%) to AS+MQ.
Conclusions: DHA/PQP was a highly efficacious drug for P. falciparum malaria in areas where multidrug parasites are prevalent. The DHA/PQP combination can play an important role in the first-line treatment of uncomplicated falciparum malaria.
Trial registration: Controlled-Trials.com ISRCTN81306618.
Conflict of interest statement
Figures
References
-
- Chen L, Qu F, Zhou Y. Field observations on the anti- malarial piperaquine. Chin Med J. 1982;95:281–286. - PubMed
-
- Hein T, Dolecek C, Mai P, Dung N, Truong N, et al. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet. 2004;363:18–22. - PubMed
-
- World Health Organization. Report of a technical consultation. Geneva: World Health Organization, Apr 4-5; 2001. Anti-malarial drug combination therapy. WHO/CDS/RBM/2001.
-
- Tip N, Trung T, Tan T, Phuc N. A field trial for efficacy of CV8 in treatment of uncomplicated falciparum malaria. J Malaria Parasit Dis Cont. 2001:45–51.
-
- Tien N, Uyen T, Huong D, Nhan D. Efficacy of CV8 for treatment of drug-resistant falciparum malaria. J Malaria Parasit Dis Cont. 2002:37–40.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials