Epstein-Barr virus interferes with the amplification of IFNalpha secretion by activating suppressor of cytokine signaling 3 in primary human monocytes
- PMID: 20689596
- PMCID: PMC2912847
- DOI: 10.1371/journal.pone.0011908
Epstein-Barr virus interferes with the amplification of IFNalpha secretion by activating suppressor of cytokine signaling 3 in primary human monocytes
Abstract
Background: Epstein-Barr virus is recognized to cause lymphoproliferative disorders and is also associated with cancer. Evidence suggests that monocytes are likely to be involved in EBV pathogenesis, especially due to a number of cellular functions altered in EBV-infected monocytes, a process that may affect efficient host defense. Because type I interferons (IFNs) are crucial mediators of host defense against viruses, we investigated the effect of EBV infection on the IFNalpha pathway in primary human monocytes.
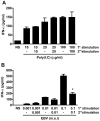
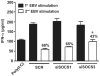
Methodology/principal findings: Infection of monocytes with EBV induced IFNalpha secretion but inhibited the positive feedback loop for the amplification of IFNalpha. We showed that EBV infection induced the expression of suppressor of cytokine signaling 3 (SOCS3) and, to a lesser extent, SOCS1, two proteins known to interfere with the amplification of IFNalpha secretion mediated by the JAK/STAT signal transduction pathway. EBV infection correlated with a blockage in the activation of JAK/STAT pathway members and affected the level of phosphorylated IFN regulatory factor 7 (IRF7). Depletion of SOCS3, but not SOCS1, by small interfering RNA (siRNA) abrogated the inhibitory effect of EBV on JAK/STAT pathway activation and significantly restored IFNalpha secretion. Finally, transfection of monocytes with the viral protein Zta caused the upregulation of SOCS3, an event that could not be recapitulated with mutated Zta.
Conclusions/significance: We propose that EBV protein Zta activates SOCS3 protein as an immune escape mechanism that both suppresses optimal IFNalpha secretion by human monocytes and favors a state of type I IFN irresponsiveness in these cells. This immunomodulatory effect is important to better understand the aspects of the immune response to EBV.
Conflict of interest statement
Figures





References
-
- Straus SE, Cohen JI, Tosato G, Meier J. NIH conference. Epstein-Barr virus infections: biology, pathogenesis, and management. Ann Intern Med. 1993;118:45–58. - PubMed
-
- Kasahara Y, Yachie A. Cell type specific infection of Epstein-Barr virus (EBV) in EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Crit Rev Oncol Hematol. 2002;44:283–294. - PubMed
-
- Savard M, Gosselin J. Epstein-Barr virus immunossuppression of innate immunity mediated by phagocytes. Virus Res. 2006;119:134–145. - PubMed
-
- Gosselin J, Menezes J, D'Addario M, Hiscott J, Flamand L, et al. Inhibition of tumor necrosis factor-alpha transcription by Epstein-Barr virus. Eur J Immunol. 1991;21:203–208. - PubMed
-
- Jabs WJ, Wagner HJ, Maurmann S, Hennig H, Kreft B. Inhibition of macrophage inflammatory protein-1 alpha production by Epstein-Barr virus. Blood. 2002;99:1512–1516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

