Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD
- PMID: 20689641
- PMCID: PMC2915539
- DOI: 10.2147/dddt.s7667
Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD
Abstract
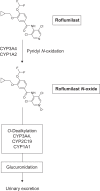
In April 2010, the European Medicines Agency Committee for Medicinal Products for Human Use recommended approval of roflumilast, a selective phosphodiesterase 4 inhibitor, for the "maintenance treatment of severe chronic obstructive pulmonary disease (COPD, FEV(1) postbronchodilator less than 50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as add-on to bronchodilator treatment". This decision was based, in part, on the results of several large, international, multicenter, randomized, placebo-controlled trials of either six or 12 months' duration that had been undertaken in COPD patients. Roflumilast 500 mug daily improved lung function and reduced exacerbations in patients with more severe COPD, especially those with chronic bronchitis, frequent exacerbations, or who required frequent rescue inhaler therapy in the placebo-controlled trials. It also improved lung function and reduced exacerbations in patients with moderately severe COPD treated with salmeterol or tiotropium. Advantages of roflumilast over inhaler therapy are that it is an oral tablet and only needs to be taken once daily. While taking roflumilast, the most common adverse effects patients experienced were gastrointestinal upset and headache. Weight loss, averaging 2.2 kg, occurred in patients treated with roflumilast. Patients taking roflumilast were more likely to drop out of the trials than patients in the control groups. Patients who discontinued therapy usually did so during the first few weeks and were more likely to have experienced gastrointestinal side effects. Roflumilast is the first selective phosphodiesterase 4 inhibitor and will offer physicians another treatment option for patients with more severe COPD.
Keywords: chronic obstructive pulmonary disease; exacerbation; phosphodiesterase 4 inhibitor; roflumilast.
Figures
References
-
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am Respir Crit Care Med. 2007;176(6):532–555. - PubMed
-
- Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance: United States, 1971–2000. MMWR CDC Surveill Summ. 2002;51(6):1–16. - PubMed
-
- Connors AF, Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med. 1996;154(4 Pt1):959–967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous