Biocatalytic asymmetric formation of tetrahydro-β-carbolines
- PMID: 20689670
- PMCID: PMC2913883
- DOI: 10.1016/j.tetlet.2010.06.075
Biocatalytic asymmetric formation of tetrahydro-β-carbolines
Abstract
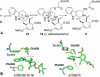
Strictosidine synthase triggers the formation of strictosidine from tryptamine and secologanin, thereby generating a carbon-carbon bond and a new stereogenic center. Strictosidine contains a tetrahydro-β-carboline moiety - an important N-heterocyclic framework found in a range of natural products and synthetic pharmaceuticals. Stereoselective methods to produce tetrahydro-β-carboline enantiomers are greatly valued. We report that strictosidine synthase from Ophiorrhiza pumila utilizes a range of simple achiral aldehydes and substituted tryptamines to form highly enantioenriched (ee >98%) tetrahydro-β-carbolines via a Pictet-Spengler reaction. This is the first example of aldehyde substrate promiscuity in the strictosidine synthase family of enzymes and represents a first step towards developing a general biocatalytic strategy to access chiral tetrahydro-β-carbolines.
Figures
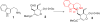
Similar articles
-
Asymmetric Synthesis of (R)-1-Alkyl-Substituted Tetrahydro-ß-carbolines Catalyzed by Strictosidine Synthases.Angew Chem Int Ed Engl. 2018 Aug 13;57(33):10683-10687. doi: 10.1002/anie.201803372. Epub 2018 Jun 21. Angew Chem Int Ed Engl. 2018. PMID: 29852524 Free PMC article.
-
Inverted Binding of Non-natural Substrates in Strictosidine Synthase Leads to a Switch of Stereochemical Outcome in Enzyme-Catalyzed Pictet-Spengler Reactions.J Am Chem Soc. 2020 Jan 15;142(2):792-800. doi: 10.1021/jacs.9b08704. Epub 2020 Jan 7. J Am Chem Soc. 2020. PMID: 31909617 Free PMC article.
-
Asymmetric Biocatalytic Synthesis of 1-Aryltetrahydro-β-carbolines Enabled by "Substrate Walking".Chemistry. 2020 Dec 9;26(69):16281-16285. doi: 10.1002/chem.202004449. Epub 2020 Nov 3. Chemistry. 2020. PMID: 33017078 Free PMC article.
-
Application of Pictet-Spengler Reaction to Indole-Based Alkaloids Containing Tetrahydro-β-carboline Scaffold in Combinatorial Chemistry.ACS Comb Sci. 2017 Apr 10;19(4):199-228. doi: 10.1021/acscombsci.6b00184. Epub 2017 Mar 17. ACS Comb Sci. 2017. PMID: 28276678 Review.
-
The Chiral Pool in the Pictet-Spengler Reaction for the Synthesis of β-Carbolines.Molecules. 2016 May 27;21(6):699. doi: 10.3390/molecules21060699. Molecules. 2016. PMID: 27240334 Free PMC article. Review.
Cited by
-
Flavones: From Biosynthesis to Health Benefits.Plants (Basel). 2016 Jun 21;5(2):27. doi: 10.3390/plants5020027. Plants (Basel). 2016. PMID: 27338492 Free PMC article. Review.
-
Uncovering the Mechanism of Azepino-Indole Skeleton Formation via Pictet-Spengler Reaction by Strictosidine Synthase: A Quantum Chemical Investigation.ChemistryOpen. 2023 Jun;12(6):e202300043. doi: 10.1002/open.202300043. Epub 2023 May 30. ChemistryOpen. 2023. PMID: 37248801 Free PMC article.
-
Building Bridges: Biocatalytic C-C-Bond Formation toward Multifunctional Products.ACS Catal. 2016 Jul 1;6(7):4286-4311. doi: 10.1021/acscatal.6b00758. Epub 2016 Jun 8. ACS Catal. 2016. PMID: 27398261 Free PMC article. Review.
-
Enzyme catalysed Pictet-Spengler formation of chiral 1,1'-disubstituted- and spiro-tetrahydroisoquinolines.Nat Commun. 2017 Apr 3;8:14883. doi: 10.1038/ncomms14883. Nat Commun. 2017. PMID: 28368003 Free PMC article.
-
Total Synthesis of (-)-Strictosidine and Interception of Aryne Natural Product Derivatives "Strictosidyne" and "Strictosamidyne".J Am Chem Soc. 2021 May 19;143(19):7471-7479. doi: 10.1021/jacs.1c02004. Epub 2021 May 6. J Am Chem Soc. 2021. PMID: 33955226 Free PMC article.
References
-
- Taylor MS, Jacobsen EN. J. Am. Chem. Soc. 2004;126:10558–10559. - PubMed
- Seayad J, Seayad AM, List B. J. Am. Chem. Soc. 2006;128:1086–1087. - PubMed
- Raheem IT, Thiara PS, Peterson EA, Jacobsen EN. J. Am. Chem. Soc. 2007;129:13404–13405. - PubMed
- Wanner MJ, van der Haas RN, de Cuba KR, van Maarseveen JH, Hiemstra H. Angew. Chem. Int. Ed. Engl. 2007;46:7485–7487. - PubMed
- Sewgobind NV, Wanner MJ, Ingemann S, de Gelder R, van Maarseveen JH, Hiemstra H. J. Org. Chem. 2008;73:6405–6408. - PubMed
- Klausen RS, Jacobsen EN. Org. Lett. 2009;11:887–890. - PMC - PubMed
- Bou-Hamdan FR, Leighton JL. Angew. Chem. Int. Ed. Engl. 2009;48:2403–2406. - PubMed
-
- O'Connor SE, Maresh JJ. Nat. Prod. Rep. 2006;23:532–547. - PubMed
-
- Faber K. Biotransformations in Organic Synthesis. 5th ed. Springer; 2008.
- Bornscheuer UT, Kazlauskas RJ. Hydrolases in Organic Synthesis. 2nd ed. Wiley~VCH; 2006.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
