FDG PET in the Evaluation of Parkinson's Disease
- PMID: 20689674
- PMCID: PMC2913894
- DOI: 10.1016/j.cpet.2009.12.004
FDG PET in the Evaluation of Parkinson's Disease
Abstract
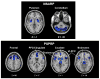
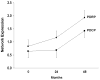
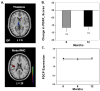
Network analysis of (18)F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is an innovative approach for the study of in movement disorders, such as Parkinson's disease (PD). Spatial covariance analysis of imaging data acquired from PD patients has revealed characteristic regional patterns associated with the motor and cognitive features of disease. Quantification of pattern expression in individual patients can be used for diagnosis, assessment of disease severity, and evaluation of novel medical and surgical therapies. Identification of disease-specific patterns in other parkinsonian syndromes, such as multiple system atrophy and progressive supranuclear palsy, has improved diagnostic accuracy in patients with difficult to diagnose parkinsonism. Further developments of these techniques are likely to enhance the role of functional imaging in investigating underlying abnormalities and potential new therapies in these neurodegenerative diseases.
Figures

References
-
- de Rijk MC, Breteler MM, Graveland GA, et al. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology. 1995;45(12):2143–6. - PubMed
-
- Hely MA, Morris JG, Reid WG, et al. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20(2):190–9. - PubMed
-
- Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–44. - PubMed
-
- Hughes AJ, Daniel SE, Ben-Shlomo Y, et al. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(Pt 4):861–70. - PubMed
-
- O’Sullivan SS, Massey LA, Williams DR, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131(Pt 5):1362–72. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
