The Catalytic Function of Nonheme Iron (III) Complex for Hydrocarbon Oxidation
- PMID: 20689711
- PMCID: PMC2905942
- DOI: 10.1155/2010/861892
The Catalytic Function of Nonheme Iron (III) Complex for Hydrocarbon Oxidation
Abstract
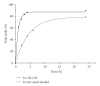
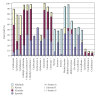
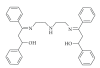
A detailed catalytic study of LFe(III)Cl (where L = 3-{2-[2-(3-hydroxy-1,3-diphenyl-allylideneamino)-ethylamino]-ethylimino}-1,3-diphenyl-propen-1-ol) for hydrocarbon oxidation was carried out, focusing on the role of solvent, atmospheric dioxygen, and oxidant on catalytic efficiency. The data showed that LFe(III)Cl catalyst was efficient in homogeneous hydrocarbon oxidations providing significant yields. Moreover, tert-BuOOH provided comparable oxidation yields with H(2)O(2), slightly favoring the formation of alcohols and ketones versus epoxides. Dioxygen intervened in the catalytic reaction, influencing the nature of oxidation products. The polarity of solvent strongly influenced the reaction rates and the nature of oxidation products. A mechanistic model is postulated assuming that LFe(III)Cl functions via the formation of iron-hydroperoxo-species, followed by a radical-based mechanistic path.
Figures



Similar articles
-
Synthetic mononuclear nonheme iron-oxygen intermediates.Acc Chem Res. 2015 Aug 18;48(8):2415-23. doi: 10.1021/acs.accounts.5b00218. Epub 2015 Jul 23. Acc Chem Res. 2015. PMID: 26203519
-
Bioinspired Nonheme Iron Catalysts for C-H and C═C Bond Oxidation: Insights into the Nature of the Metal-Based Oxidants.Acc Chem Res. 2015 Sep 15;48(9):2612-21. doi: 10.1021/acs.accounts.5b00053. Epub 2015 Aug 17. Acc Chem Res. 2015. PMID: 26280131
-
Biomimetic aryl hydroxylation derived from alkyl hydroperoxide at a nonheme iron center. Evidence for an Fe(IV)=O oxidant.J Am Chem Soc. 2003 Feb 26;125(8):2113-28. doi: 10.1021/ja028478l. J Am Chem Soc. 2003. PMID: 12590539
-
Dioxygen Activation by Nonheme Diiron Enzymes: Diverse Dioxygen Adducts, High-Valent Intermediates, and Related Model Complexes.Chem Rev. 2018 Mar 14;118(5):2554-2592. doi: 10.1021/acs.chemrev.7b00457. Epub 2018 Feb 5. Chem Rev. 2018. PMID: 29400961 Free PMC article. Review.
-
Bio-inspired Nonheme Iron Oxidation Catalysis: Involvement of Oxoiron(V) Oxidants in Cleaving Strong C-H Bonds.Angew Chem Int Ed Engl. 2020 May 4;59(19):7332-7349. doi: 10.1002/anie.201906551. Epub 2020 Mar 2. Angew Chem Int Ed Engl. 2020. PMID: 31373120 Review.
References
-
- Furuhashi K. Biological routes to optically active epoxides. In: Collins AN, Sheldrake GN, Crosby J, editors. Chirality in Industry. London, UK: John Wiley & Sons; 1992. pp. 167–186.
-
- Sundermeier U, Döbler C, Beller M. Recent developments in the osmium-catalyzed dihydroxylation of olefins. In: Bäckvall JE, editor. Modern Oxidation Methods. Weinheim, Germany: Wiley-VCH; 2004. p. 1.
-
- Costas M, Mehn MP, Jensen MP, Que L., Jr. Dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chemical Reviews. 2004;104(2):939–986. - PubMed
-
- Mas-Ballesté R, Costas M, Van Den Berg T, Que L., Jr. Ligand topology effects on olefin oxidations by bio-inspired [FeII(N2Py2)] catalysts. Chemistry—A European Journal. 2006;12(28):7489–7500. - PubMed
-
- Shikama K. The molecular mechanism of autoxidation for myoglobin and hemoglobin: a venerable puzzle. Chemical Reviews. 1998;98(4):1357–1373. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

