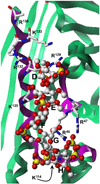
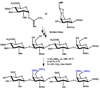
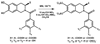
Chemical Sulfation of Small Molecules - Advances and Challenges
- PMID: 20689724
- PMCID: PMC2913517
- DOI: 10.1016/j.tet.2010.02.015
Chemical Sulfation of Small Molecules - Advances and Challenges
Figures
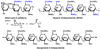








Similar articles
-
Chemical approaches to the sulfation of small molecules: current progress and future directions.Essays Biochem. 2024 Dec 4;68(4):449-466. doi: 10.1042/EBC20240001. Essays Biochem. 2024. PMID: 38958528 Free PMC article. Review.
-
Chemical Methods for N- and O-Sulfation of Small Molecules, Amino Acids and Peptides.Chembiochem. 2020 Apr 1;21(7):938-942. doi: 10.1002/cbic.201900673. Epub 2020 Jan 3. Chembiochem. 2020. PMID: 31692230
-
Exploiting Chemical Protein Synthesis to Study the Role of Tyrosine Sulfation on Anticoagulants from Hematophagous Organisms.Acc Chem Res. 2023 Oct 3;56(19):2688-2699. doi: 10.1021/acs.accounts.3c00388. Epub 2023 Sep 14. Acc Chem Res. 2023. PMID: 37708351
-
Human gastrointestinal sulfotransferases: identification and distribution.Toxicol Appl Pharmacol. 2003 Mar 15;187(3):186-97. doi: 10.1016/s0041-008x(02)00073-x. Toxicol Appl Pharmacol. 2003. PMID: 12662902
-
Revealing the functional roles of tyrosine sulfation using synthetic sulfopeptides and sulfoproteins.Curr Opin Chem Biol. 2020 Oct;58:72-85. doi: 10.1016/j.cbpa.2020.05.007. Epub 2020 Aug 7. Curr Opin Chem Biol. 2020. PMID: 32777686 Review.
Cited by
-
Allosteric Partial Inhibition of Monomeric Proteases. Sulfated Coumarins Induce Regulation, not just Inhibition, of Thrombin.Sci Rep. 2016 Apr 7;6:24043. doi: 10.1038/srep24043. Sci Rep. 2016. PMID: 27053426 Free PMC article.
-
Designing Smaller, Synthetic, Functional Mimetics of Sulfated Glycosaminoglycans as Allosteric Modulators of Coagulation Factors.J Med Chem. 2023 Apr 13;66(7):4503-4531. doi: 10.1021/acs.jmedchem.3c00132. Epub 2023 Mar 31. J Med Chem. 2023. PMID: 37001055 Free PMC article. Review.
-
Sulfated and Phosphorylated Agarose as Biomaterials for a Biomimetic Paradigm for FGF-2 Release.Biomimetics (Basel). 2024 Dec 30;10(1):12. doi: 10.3390/biomimetics10010012. Biomimetics (Basel). 2024. PMID: 39851728 Free PMC article.
-
Nonsulfated, cinnamic acid-based lignins are potent antagonists of HSV-1 entry into cells.Biomacromolecules. 2010 May 10;11(5):1412-6. doi: 10.1021/bm100161u. Biomacromolecules. 2010. PMID: 20411926 Free PMC article.
-
Designing nonsaccharide, allosteric activators of antithrombin for accelerated inhibition of factor Xa.J Med Chem. 2011 Sep 8;54(17):6125-38. doi: 10.1021/jm2008387. Epub 2011 Aug 12. J Med Chem. 2011. PMID: 21800826 Free PMC article.
References
-
- Roy AB. Trends Biochem. Sci. 1976;1:N233–N234.
-
- Zamek-Gliszczynski MJ, Hoffmaster KA, Nezasa K, Tallman MN, Brouwer KL. Eur. J. Pharm. Sci. 2006;27:447–486. - PubMed
-
- Falany CN. FASEB J. 1997;11:206–216. - PubMed
-
- Bowman KG, Bertozzi CR. Chem. Biol. 1999;6:R9–R22. - PubMed
-
- Grunwell JR, Bertozzi CR. Biochemistry. 2002;41:13117–13126. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
