Back to the future: studying cholera pathogenesis using infant rabbits
- PMID: 20689747
- PMCID: PMC2912669
- DOI: 10.1128/mBio.00047-10
Back to the future: studying cholera pathogenesis using infant rabbits
Abstract
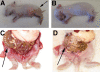
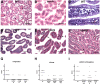
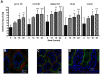
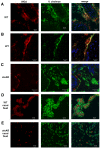
Cholera is a severe diarrheal disease, caused by Vibrio cholerae, for which there has been no reproducible, nonsurgical animal model. Here, we report that orogastric inoculation of V. cholerae into 3-day-old rabbits pretreated with cimetidine led to lethal, watery diarrhea in virtually all rabbits. The appearance and chemical composition of the rabbit diarrheal fluid were comparable to those of the "rice-water stool" produced by cholera patients. As in humans, V. cholerae mutants that do not produce cholera toxin (CT) and toxin-coregulated pilus (TCP) did not induce cholera-like disease in rabbits. CT induced extensive exocytosis of mucin from intestinal goblet cells, and wild-type V. cholerae was predominantly found in close association with mucin. Large aggregates of mucin-embedded V. cholerae were observed, both attached to the epithelium and floating within the diarrheal fluid. These findings suggest that CT-dependent mucin secretion significantly influences V. cholerae's association with the host intestine and its exit from the intestinal tract. Our model should facilitate identification and analyses of factors that may govern V. cholerae infection, survival, and transmission, such as mucin. In addition, our results using nontoxigenic V. cholerae suggest that infant rabbits will be useful for study of the reactogenicity of live attenuated-V. cholerae vaccines.
Figures


Similar articles
-
Reactogenicity of live-attenuated Vibrio cholerae vaccines is dependent on flagellins.Proc Natl Acad Sci U S A. 2010 Mar 2;107(9):4359-64. doi: 10.1073/pnas.0915164107. Epub 2010 Feb 16. Proc Natl Acad Sci U S A. 2010. PMID: 20160087 Free PMC article.
-
Infant Rabbit Model for Diarrheal Diseases.Curr Protoc Microbiol. 2015 Aug 3;38:6A.6.1-15. doi: 10.1002/9780471729259.mc06a06s38. Curr Protoc Microbiol. 2015. PMID: 26237109 Free PMC article.
-
Single Nucleotide Polymorphisms in Regulator-Encoding Genes Have an Additive Effect on Virulence Gene Expression in a Vibrio cholerae Clinical Isolate.mSphere. 2016 Sep 21;1(5):e00253-16. doi: 10.1128/mSphere.00253-16. eCollection 2016 Sep-Oct. mSphere. 2016. PMID: 27668288 Free PMC article.
-
Cholera: pathophysiology and emerging therapeutic targets.Future Med Chem. 2013 May;5(7):781-98. doi: 10.4155/fmc.13.42. Future Med Chem. 2013. PMID: 23651092 Review.
-
Modulation of Host-Microbe Metabolism by Cholera Toxin.Infect Immun. 2023 May 16;91(5):e0043522. doi: 10.1128/iai.00435-22. Epub 2023 Apr 6. Infect Immun. 2023. PMID: 37022166 Free PMC article. Review.
Cited by
-
Effects of tcpB Mutations on Biogenesis and Function of the Toxin-Coregulated Pilus, the Type IVb Pilus of Vibrio cholerae.J Bacteriol. 2016 Sep 22;198(20):2818-28. doi: 10.1128/JB.00309-16. Print 2016 Oct 15. J Bacteriol. 2016. PMID: 27481929 Free PMC article.
-
RpoS and quorum sensing control expression and polar localization of Vibrio cholerae chemotaxis cluster III proteins in vitro and in vivo.Mol Microbiol. 2015 Aug;97(4):660-75. doi: 10.1111/mmi.13053. Epub 2015 Jun 6. Mol Microbiol. 2015. PMID: 25989366 Free PMC article.
-
The Vibrio cholerae trh gene is coordinately regulated in vitro with type III secretion system genes by VttR(A)/VttR(B) but does not contribute to Caco2-BBE cell cytotoxicity.Infect Immun. 2012 Dec;80(12):4444-55. doi: 10.1128/IAI.00832-12. Epub 2012 Oct 8. Infect Immun. 2012. PMID: 23045478 Free PMC article.
-
Transposon-insertion sequencing screens unveil requirements for EHEC growth and intestinal colonization.PLoS Pathog. 2019 Aug 12;15(8):e1007652. doi: 10.1371/journal.ppat.1007652. eCollection 2019 Aug. PLoS Pathog. 2019. PMID: 31404118 Free PMC article.
-
Animal models for dissecting Vibrio cholerae intestinal pathogenesis and immunity.Curr Opin Microbiol. 2022 Feb;65:1-7. doi: 10.1016/j.mib.2021.09.007. Epub 2021 Oct 22. Curr Opin Microbiol. 2022. PMID: 34695646 Free PMC article. Review.
References
-
- Lacey S. W. 1995. Cholera: calamitous past, ominous future. Clin. Infect. Dis. 20:1409–1419 - PubMed
-
- Sack D. A., Sack R. B., Chaignat C. L. 2006. Getting serious about cholera. N. Engl. J. Med. 355:649–651 - PubMed
-
- WHO 2009. Cholera: global surveillance summary, 2008. Wkly. Epidemiol. Rec. 84:309–324 - PubMed
-
- Sack D. A., Sack R. B., Nair G. B., Siddique A. K. 2004. Cholera. Lancet 363:223–233 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

