KinD is a checkpoint protein linking spore formation to extracellular-matrix production in Bacillus subtilis biofilms
- PMID: 20689749
- PMCID: PMC2912670
- DOI: 10.1128/mBio.00035-10
KinD is a checkpoint protein linking spore formation to extracellular-matrix production in Bacillus subtilis biofilms
Abstract
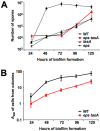
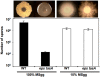
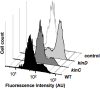
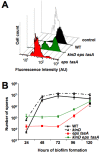
Bacillus subtilis cells form multicellular biofilm communities in which spatiotemporal regulation of gene expression occurs, leading to differentiation of multiple coexisting cell types. These cell types include matrix-producing and sporulating cells. Extracellular matrix production and sporulation are linked in that a mutant unable to produce matrix is delayed for sporulation. Here, we show that the delay in sporulation is not due to a growth advantage of the matrix-deficient mutant under these conditions. Instead, we show that the link between matrix production and sporulation is through the Spo0A signaling pathway. Both processes are regulated by the phosphorylated form of the master transcriptional regulator Spo0A. When cells have low levels of phosphorylated Spo0A (Spo0A~P), matrix genes are expressed; however, at higher levels of Spo0A~P, sporulation commences. We have found that Spo0A~P levels are maintained at low levels in the matrix-deficient mutant, thereby delaying expression of sporulation-specific genes. This is due to the activity of one of the components of the Spo0A phosphotransfer network, KinD. A deletion of kinD suppresses the sporulation defect of matrix mutants, while its overproduction delays sporulation. Our data indicate that KinD displays a dual role as a phosphatase or a kinase and that its activity is linked to the presence of extracellular matrix in the biofilms. We propose a novel role for KinD in biofilms as a checkpoint protein that regulates the onset of sporulation by inhibiting the activity of Spo0A until matrix, or a component therein, is sensed.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
